Making sodium-ion batteries that last
Lithium-ion batteries have become essential in everyday technology. But these power sources can explode under certain circumstances and are not ideal for grid-scale energy storage. Sodium-ion batteries are potentially a safer and less expensive alternative, but current versions don't last long enough yet for practical use. Now, scientists have developed an anode material that enables sodium-ion batteries to perform at high capacity over hundreds of cycles, according to their report in the journal ACS Nano.
For years, scientists have considered sodium-ion batteries a safer and lower-cost candidate for large-scale energy storage than lithium-ion. But so far, sodium-ion batteries have not operated at high capacity for long-term use. Lithium and sodium have similar properties in many ways, but sodium ions are much larger than lithium ions. This size difference leads to the rapid deterioration of a key battery component. Meilin Liu, Chenghao Yang and colleagues wanted to find an anode material that would give sodium-ion batteries a longer life.
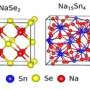
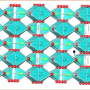
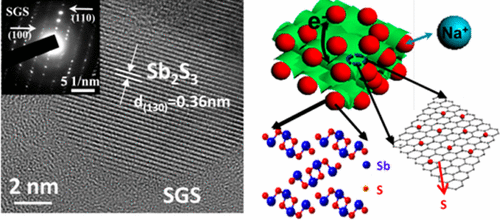
The researchers developed a simple approach to making a high-performance anode material by binding an antimony-based mineral onto sulfur-doped graphene sheets. Incorporating the anode into a sodium-ion battery allowed it to perform at 83 percent capacity over 900 cycles. The researchers say this is the best reported performance for a sodium-ion battery with an antimony-based anode material. To ultimately commercialize their technology, they would need to scale up battery fabrication while maintaining its high performance.
More information: Xunhui Xiong et al. Enhancing Sodium Ion Battery Performance by Strongly Binding Nanostructured SbSon Sulfur-Doped Graphene Sheets, ACS Nano (2016). DOI: 10.1021/acsnano.6b05653
Abstract
Sodium ion batteries (SIBs) have been considered a promising alternative to lithium ion batteries for large-scale energy storage. However, their inferior electrochemical performances, especially cyclability, become the major challenge for further development of SIBs. Large volume change and sluggish diffusion kinetics are generally considered to be responsible for the fast capacity degradation. Here we report the strong chemical bonding of nanostructured Sb2S3 on sulfur-doped graphene sheets (Sb2S3/SGS) that enables a stable capacity retention of 83% for 900 cycles with high capacities and excellent rate performances. To the best of our knowledge, the cycling performance of the Sb2S3/SGS composite is superior to those reported for any other Sb-based materials for SIBs. Computational calculations demonstrate that sulfur-doped graphene (SGS) has a stronger affinity for Sb2S3 and the discharge products than pure graphene, resulting in a robust composite architecture for outstanding cycling stability. Our study shows a feasible and effective way to solve the long-term cycling stability issue for SIBs.
Journal information: ACS Nano
Provided by American Chemical Society