Large, crystalline lipid scaffolds bring new possibilities to protein, drug research

Proteins and drugs are often attached to lipids to promote crystallization or ensure delivery to targeted tissues within the body, but only the smallest proteins and molecules fit within these fat structures. A new study reveals a lipid structure that can support much larger proteins and molecules than before, potentially increasing the variety of drugs that can be attached to these fat molecules.
The new findings are published in the Proceedings of the National Academy of Sciences.
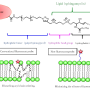
Lipids are soft, gel-like materials that can organize themselves into materials having tiny pores that proteins and drugs can squeeze into for research and medical purposes. Although lipids make an excellent substrate for drug delivery, their small pores limit the variety of medicines that can hitch a ride, leaving behind the larger molecule drugs like insulin, said professor of materials science and engineering and study leader Cecilia Leal .
Lipids also act as scaffolds to prop up proteins for atomic structure analysis. Normally, proteins are too floppy to stand up to the types of X-ray analysis used to observe them, but lipids are just crystalline enough to hold them up for researchers to see inside of them. However, this only works for small-protein molecules.
"We found a way to crystallize a 3-D lipid structure with pore spaces that are five times the size of a regular lipid," Leal said. "Now we are able, in principle, to crystallize much bigger proteins as well as encapsulate much larger drug molecules than ever before."
Much of the significance of this research is a result of the new method the group used to prepare the lipid scaffolds.
"The materials are old; there is nothing new there," Leal said. "What we did differently was to find a new way to prepare the lipid cocktail to produce this unique material."In fact, Leal's group discovered this new preparation technique by accident.
"One of my students, a co-author of this study, had rushed the preparation process and later realized his mistake when he went to examine the lipid pore sizes in the material he had just produced," Leal said. "The pores were much larger than they should have been. We did not think that the large pores would remain stable, but they did, and the process is fully reproducible. Furthermore, the large porous lipid structure developed as a crystal, which is unusual for these soft materials. "
The study's findings are interesting from a scientific viewpoint, even paradigm-shifting, Leal said, because researchers do not associate lipid membranes with crystallinity, which is commonly found in hard materials. The group hopes that this will lead more scientists to look at these materials in a new way.
"However, it is the application of material to the study of proteins and drug delivery that will have the most impact," Leal said.
The National Institutes of Health and the Office of Naval Research supported this research.
More information: Super-swelled lyotropic single crystals, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1710774114 , www.pnas.org/content/early/201 … /1710774114.abstract
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Illinois at Urbana-Champaign