Team develops sustainable, high energy density battery

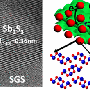
Researchers at The City College of New York-based CUNY Energy Institute announce the development of a novel low cost, rechargeable, high energy density battery that makes the widespread use of solar and wind power possible in the future. It is based on manganese dioxide (MnO2), an abundant, safe and non-toxic material.
In a paper in the journal Nature Communications, the scientists report that the uniqueness of the battery is that it is able to achieve both high cycle life and high areal capacity. Achieving high areal capacity is critical for packing a lot of battery electrodes together into a battery case. In essence a high areal capacity is required to build a real, practical battery, as opposed to a small toy battery.
Past researchers have achieved either high cycle life or high areal capacity, but never both together, the team notes.
The innovation that makes this possible is intercalating copper (Cu) into bismuth-modified δ-MnO2, which is called birnessite. The latter was discovered by Ford Motor Company in the 1980s, but it was never known how to use it at high areal capacity. This was later discovered at the CUNY Energy Institute by a team led by Sanjoy Banerjee, Distinguished Professor and director of the Institute.
The battery is intended for use at the scale of the power grid. This would make widespread use of solar and wind power possible.
More information: Gautam G. Yadav et al. Regenerable Cu-intercalated MnO2 layered cathode for highly cyclable energy dense batteries, Nature Communications (2017). DOI: 10.1038/ncomms14424
Journal information: Nature Communications
Provided by City College of New York