Super stable garnet ceramics may be ideal for high-energy lithium batteries
Scientists at the Department of Energy's Oak Ridge National Laboratory have discovered exceptional properties in a garnet material that could enable development of higher-energy battery designs.
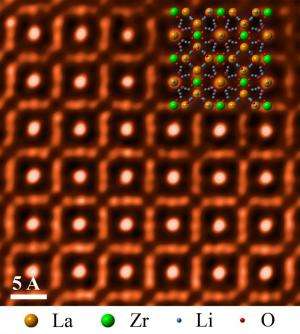
The ORNL-led team used scanning transmission electron microscopy to take an atomic-level look at a cubic garnet material called LLZO. The researchers found the material to be highly stable in a range of aqueous environments, making the compound a promising component in new battery configurations.
Researchers frequently seek to improve a battery's energy density by using a pure lithium anode, which offers the highest known theoretical capacity, and an aqueous electrolyte that can speedily transport lithium. The ORNL scientists believe the LLZO would be an ideal separator material, which is crucial.
"Many novel batteries adopt these two features [lithium anode and aqueous electrolyte], but if you integrate both into a single battery, a problem arises because the water is very reactive when in direct contact with lithium metal," said ORNL postdoctoral associate Cheng Ma, first author on the team's study published in Angewandte Chemie. "The reaction is very violent, which is why you need a protective layer around the lithium."
Battery designers can use a solid electrolyte separator to shield the lithium, but their options are limited. Even the primary separator of choice, known as LAPT or LISICON, tends to break down under normal battery operating conditions.
"Researchers have searched for a suitable solid electrolyte separator material for years," said ORNL's Miaofang Chi, the study's lead author. "The requirements for this type of material are very strict. It must be compatible with the lithium anode because lithium is reactive, and it also has to be stable over a wide pH range, because you can have an alkaline environment—especially with lithium air batteries."
The researchers used atomic resolution imaging to monitor structural changes in LLZO after the samples' immersion in a range of aqueous solutions. The team's observations showed that the compound remained structurally stable over time across neutral and extremely alkaline environments.
"This solid electrolyte separator remains stable even for a pH value higher than 14," Ma said. "It gives battery designers more options for the selection of aqueous solutions and the catholyte." Catholyte is the portion of the electrolyte close to the cathode.
In lithium-air batteries, for instance, researchers have previously tried to avoid the degradation of the separator by diluting the aqueous solutions, which only makes the battery heavier and bulkier. With this new type of solid electrolyte separator, there is no need to dilute the aqueous electrolyte, so it indirectly increases the battery's energy density.
Higher-energy batteries are in demand for electrified transportation and electric grid energy storage applications, leading researchers to explore battery designs beyond the limits of lithium-ion technologies.
The researchers intend to continue their research by evaluating the LLZO garnet's performance in an operating battery. Coauthors are ORNL's Chengdu Liang, Karren More, Ezhiylmurugan Rangasamy, and Michigan State University's Jeffrey Sakamoto. The study is published as "Excellent Stability of a Li-Ion-Conducting Solid Electrolyte upon Reversible Li+/H+ Exchange in Aqueous Solutions."
More information: Angewandte Chemie, onlinelibrary.wiley.com/doi/10 … e.201408124/abstract
Journal information: Angewandte Chemie
Provided by Oak Ridge National Laboratory