This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research offers innovative approach to planar chiral substances
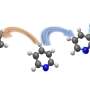
![Desymmetric esterification of [2.2]paracyclophanes. Credit: Charles University Innovative approach to planar chiral substances](https://scx1.b-cdn.net/csz/news/800a/2024/innovative-approach-to.jpg)
In a recent study, Dr. Vojtěch Dočekal and Professor Jan Veselý from the Department of Organic Chemistry at the Faculty of Science, Charles University, have unveiled a highly efficient method for the preparation of planar chiral substances. Their research, centered on organocatalytic desymmetrization, has been published in Nature Communications.
Planar chiral paracyclophanes, which consist of two appropriately substituted benzene rings connected by ethylene bridges in para positions, are versatile compounds used in synthetic chemistry as ligands or catalysts, and have applications in material science and medicine. Despite their broad utility, few studies have described methods for their preparation in enantiomerically pure form.
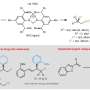
The methodology developed by the asymmetric synthesis group at Charles University relies on the highly efficient desymmetrization of prochiral paracyclophanes, substituted with two formyl groups in strategic positions.
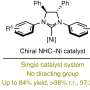
"A key transformation in this process selectively converts one of the prochiral aldehydic groups into an ester, resulting in a planar chiral product using asymmetric synthesis techniques," said Dr. Vojtěch Dočekal, co-author of the study. This asymmetric esterification has proven highly effective when using catalysis by low molecular weight chiral catalysts derived from natural amino acids, specifically through the use of N-heterocyclic carbenes.
"In addition to optimizing the methodology, the publication includes an extensive study on the scope of the developed transformation and the synthetic utility of the chiral products," concluded prof. Jan Veselý, another co-author. A detailed experimental mechanistic study is also part of the publication, revealing fascinating differences in the reactivity of the starting diformyl paracyclophanes.
More information: Vojtěch Dočekal et al, Organocatalytic desymmetrization provides access to planar chiral [2.2]paracyclophanes, Nature Communications (2024). DOI: 10.1038/s41467-024-47407-0
Journal information: Nature Communications
Provided by Charles University