September 19, 2013 feature
Molten-air battery's storage capacity among the highest of any battery type
(Phys.org) —Researchers have demonstrated a new class of high-energy battery, called a "molten-air battery," that has one of the highest storage capacities of any battery type to date. Unlike some other high-energy batteries, the molten-air battery has the advantage of being rechargeable. Although the molten electrolyte currently requires high-temperature operation, the battery is so new that the researchers hope that experimenting with different molten compositions and other characteristics will make molten-air batteries strong competitors in electric vehicles and for storing energy for the electric grid.
The researchers, Stuart Licht, Baochen Cui, Jessica Stuart, Baohui Wang, and Jason Lau, at George Washington University, have published a paper on the new molten-air battery in a recent issue of Energy & Environmental Science.
"This is the first time that a rechargeable molten-air battery has been demonstrated," Licht told Phys.org. "There have been rechargeable batteries that use molten electrolytes, but not air. For example, molten-sulfur batteries have been widely studied for electric car and grid applications. However, sulfur is twice as massive as oxygen (per electron stored) and its mass needs to be carried as part of the battery (whereas air is freely available). The molten-air batteries are the first rechargeable batteries to use a molten salt to store energy using 'free' oxygen from the air and multi-electron storage molecules."
This ability to store multiple electrons in a single molecule is one of the biggest advantages of the molten-air battery. By their nature, multiple-electron-per-molecule batteries usually have higher storage capacities compared to single-electron-per-molecule batteries, such as Li-ion batteries. The battery with the highest energy capacity to date, the vanadium boride (VB2)-air battery, can store 11 electrons per molecule. However, the VB2-air battery and many other high-capacity batteries have a serious drawback: they are not rechargeable.
Here, the researchers demonstrated that molten-air batteries offer a combination of high storage capacity and reversibility. The molten-air battery uses oxygen from the air as the cathode material, giving it the benefit of not having to carry this weight. It also has the advantage of not using any exotic catalysts or membranes. Different versions of the battery use different electrolytes, but they are all molten, i.e., melted to a liquid by a high temperature, in this case around 700-800 °C.
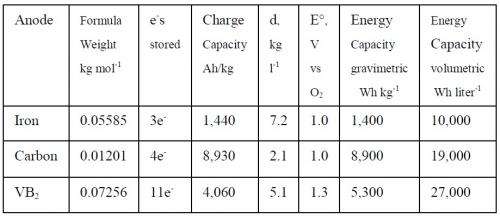
The researchers experimented with using iron, carbon, and VB2 as the molten electrolyte, demonstrating very high capacities of 10,000, 19,000, and 27,000 Wh/l, respectively. The capacities are influenced by the number of electrons that each type of molecule can store: 3 electrons for iron, 4 electrons for carbon, and 11 electrons for VB2. In comparison, the Li-air battery has an energy capacity of 6,200 Wh/l, due to its single-electron-per-molecule transfer and lower density than the other compositions.
The researchers explain that they were able to make the battery reversible by using an unusual electrolytic splitting process to function as battery "charging." For example, when the iron molten-air battery is discharged, the iron mixes with the oxygen to produce iron oxide. To charge the battery, the iron oxide is converted back into iron metal, and O2 is released into the air. The carbon and VB2 molten-air batteries recharge in a similar way, although the electrochemical properties of VB2 are not as well understood as the others.
As Licht explained, the molten electrolyte is a key to making the battery rechargeable.
"In the case of molten-air batteries, the molten electrolyte opens a pathway to recharge a wide variety of high-capacity multi-electron storage materials," he said. "These materials, while highest in capacity, are a challenge to recharge (how do you reinsert 11 electrons back into each molecule of vanadium boride?). The molten electrolyte provides an effective media that is compatible with both recharging these materials and 'free' oxygen from the air for storage. The high activity of molten electrolytes allows this charging to occur."
While the molten-air battery's high capacity and reversibility make it an attractive candidate for future energy storage applications, the researchers are continuing to improve other areas of the battery. For example, they plan to investigate other types of molten electrolytes with lower melting temperatures, increasing the voltage (a major contributor to power density and, for electric vehicles, maximum speed), and improving the energy efficiency.
"High temperature for a battery is unusual," Licht said. "However, it is not an impediment. Lower capacity, high-temperature molten electrolyte sulfur batteries have already been tested without incident in electric vehicles. No weak spot has yet appeared. The discharge current of the molten-air electrode is sufficient to yield high battery voltages, but as described in the study could be even greater when a higher surface area between the cycled air and the molten salt will be achieved."
More information:
Stuart Licht, et al. "Molten Air – A new, highest energy class of rechargeable batteries." Energy & Environmental Science. DOI: 10.1039/C3EE42654H
Also at arXiv:1307.1305 [physics.chem-ph] http://arxiv.org/abs/1307.1305
Journal information: Energy & Environmental Science
© 2013 Phys.org. All rights reserved.