Team discovers how a protein finds its way
(Phys.org) —Proteins, the workhorses of the body, can have more than one function, but they often need to be very specific in their action or they create cellular havoc, possibly leading to disease.
Scientists from the Florida campus of The Scripps Research Institute (TSRI) have uncovered how an enzyme co-factor can bestow specificity on a class of proteins with otherwise nonspecific biochemical activity.
The protein in question helps in the assembly of ribosomes, large macromolecular machines that are critical to protein production and cell growth. This new discovery expands scientists' view of the role of co-factors and suggests such co-factors could be used to modify the activity of related proteins and their role in disease.
"In ribosome production, you need to do things very specifically," said TSRI Associate Professor Katrin Karbstein, who led the study. "Adding a co-factor like Rrp5 forces these enzymes to be specific in their actions. The obvious possibility is that if you could manipulate the co-factor, you could alter protein activity, which could prove to be tremendously important."
The new study, which is being published the week of April 29, 2013, in the online Early Edition of the Proceedings of the National Academy of Sciences, sheds light on proteins called DEAD-box proteins, a provocative title actually derived from their amino acid sequence. These proteins regulate all aspects of gene expression and RNA metabolism, particularly in the production of ribosomes, and are involved in cell metabolism. The link between defects in ribosome assembly and cancer and between DEAD-box proteins and cancer is well documented.
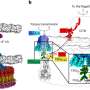
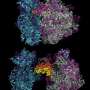
The findings show that the DEAD-box protein Rok1, needed in the production of a small ribosomal subunit, recognizes the RNA backbone, the basic structural framework of nucleic acids. The co-factor Rrp5 then gives Rok1 the ability to target a specific RNA sequence by modulating the structure of Rok1.
"Despite extensive efforts, the roles of these DEAD-box proteins in the assembly of the two ribosomal subunits remain largely unknown," Karbstein said. "Our study suggests that the solution may be to identify their cofactors first."
More information: Cofactor-dependent specificity of a DEAD-box protein , PNAS, www.pnas.org/cgi/doi/10.1073/pnas.1302577110
Journal information: Proceedings of the National Academy of Sciences
Provided by The Scripps Research Institute