Protein structure unlocks one mystery of multi-drug tolerance
The structures of key bacterial proteins have revealed one of the biochemical secrets that enables bacteria to outwit antibiotics.
In a paper published Sept. 20, 2012 in the journal Cell Reports, Duke University School of Medicine researchers and their colleagues describe the results of a series of experiments exploring multi-drug tolerance, a phenomenon that allows bacteria to become dormant and tolerate antibiotics, only to later awaken and re-infect the host. Drug tolerance is a factor in several types of stubborn, recurring infectious diseases caused by pathogenic bacteria, such E. coli, P. aeruginosa and M. tuberculosis.
"One in a million of those bacteria can become dormant for reasons we don't yet fully understand," said senior author Richard G. Brennan, Ph.D., professor and chairman of Duke's Department of Biochemistry. "And when bacteria are dormant, drugs are far, far less effective in the eradication of infection."
Multi-drug tolerance begins with a protein kinase molecule called HipA, which drives a few bacterial cells into dormancy. Eventually, these so-called "persister" cells—literally one in a million in the bacterial population—awaken to begin growing and starting the cycle of infection all over again.
"It's a very clever thing for them to do, and it's one of the underlying reasons there are so many recalcitrant infections," said Maria A. Schumacher, Ph.D., professor of biochemistry at Duke and lead author of the study.
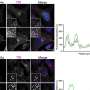
By analyzing the structural and biochemical components at work in the HipA-mediated system, the team was able to get to the bottom of how this protein works. Schumacher, the lead structural biologist in the group, used X-ray crystallography to produce an atomic-level three-dimensional structure of HipA. This structure was pivotal to understand how this simple modification affected HipA's activity.
"This protein carries out a process called phosphorylation, which affects the activities of other molecules that control dormancy," Schumacher said. "However, too much phosphorylation by HipA is likely a very bad thing for the cell, so HipA turns itself off by an unusual self-modification, which causes the modified region to become completely disordered. In fact, this normally internal part of the protein is ejected to the outside. To the best of our knowledge, this had never been seen before in any protein of this type, and it's an incredibly unusual mechanism for how it works. From a structural biology standpoint, it's a really exciting finding."
Multi-drug tolerance is not the same as the better-known state called multi-drug resistance.
"In multi-drug resistance, bacteria evolve by a number of mechanisms to become strains that are resistant to higher and higher concentrations of antibiotics," Brennan explained. "In multi-drug tolerance, the drugs don't work because the bacteria are dormant. The persister cells simply evade most drugs until it is safe for them to re-emerge and re-infect, without having mutated."
The next steps for the researchers will be to continue their structural and biochemical studies to better understand the multiple targets of the HipA protein in the cell, and how persistence is spread throughout the cell, perhaps by multiple proteins beyond just HipA. Eventually, a highly targeted drug therapy may be in sight.
"We can see how we might be able to inhibit the protein and shut down multi-drug tolerance, at least in these types of bacteria," said Brennan.
To Schumacher and Brennan, the study is an excellent example of why basic science is important for improving clinical care and developing treatments. "This is a perfect study to show how something we're doing that is very basic can have a downstream impact upon how we think about disease and disease treatments," Brennan said.
Journal information: Cell Reports
Provided by Duke University Medical Center