September 21, 2012 report
Chemists find smallest number of water molecules needed to form an ice crystal
(Phys.org)—It would seem that figuring out how many molecules of water are needed to form the smallest, or most basic ice crystal would have been discovered long ago, but that is not the case, because to measure both the size and structure of a water molecule cluster at the same time, two types of devices must be used, which up till now meant one disturbing the other, ruining the results. Now a group of chemists working at the Max Planck Institute has discovered a way around that problem and report that the minimum number of water molecules necessary to form an ice crystal is 275. In their paper published in the journal Science, the team describes the process they developed and how they obtained their results.
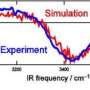
To find out the lowest number of water molecules needed to form an ice crystal, a means of detecting water molecule clusters and how many molecules are in them must be used as well as a means of determining if that cluster is a crystal structure. To measure the first, researchers use mass spectrometry. To determine the second, infrared spectroscopy is used because the frequencies that are absorbed by chemical bonds that occur in regular water clusters are slightly different than those absorbed by ice crystals.
To allow for both tests to be made at the same time, the team got around the problem of interference by doping just the surface of the water molecule clusters with sodium atoms. This allowed for mass spectrometry measurements of the cluster without disturbing its structure which meant that the team could also determine if the structure was a crystal using infrared spectroscopy.
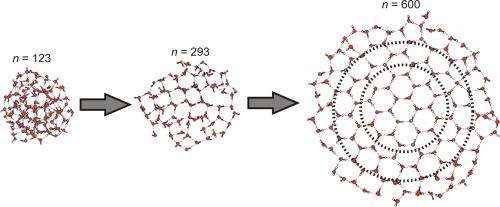
To find the smallest possible ice crystal was then a matter of brute force. The team measured water cluster sizes that ranged from 85 to 475 molecules and found that crystallization consistently occurred only after there were at least 275 molecules involved.
The team suggests that smaller ice crystals cannot form due to their not being enough space after starting from a base around a center ring made of just six water molecules which are hydrogen bonded. They also suggest their method could also be used to study the crystal structure (and the way they grow) of other materials as well.
More information: A Fully Size-Resolved Perspective on the Crystallization of Water Clusters, Science, 21 September 2012: Vol. 337 no. 6101 pp. 1529-1532. DOI: 10.1126/science.1225468
ABSTRACT
The number of water molecules needed to form the smallest ice crystals has proven challenging to pinpoint experimentally. This information would help to better understand the hydrogen-bonding interactions that account for the macroscopic properties of water. Here, we report infrared (IR) spectra of precisely size-selected (H2O)n clusters, with n ranging from 85 to 475; sodium doping and associated IR excitation–modulated photoionization spectroscopy allowed the study of this previously intractable size domain. Spectral features indicating the onset of crystallization are first observed for n = 275 ± 25; for n = 475 ± 25, the well-known band of crystalline ice around 3200 cm−1 dominates the OH-stretching region. The applied method has the potential to push size-resolved IR spectroscopy of neutral clusters more broadly to the 100- to 1000-molecule range, in which many solvents start to manifest condensed phase properties.
Journal information: Science
© 2012 Phys.org