This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Expanding click chemistry: An eco-friendly conversion process for the synthesis of sulfonyl fluorides
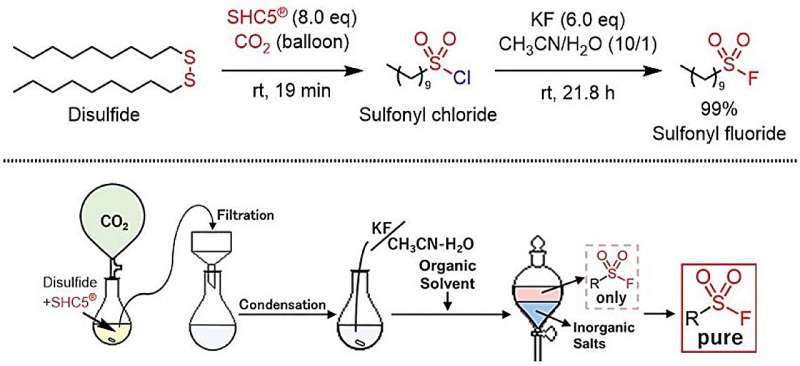
For the first time, thiols and disulfides have been converted into sulfonyl fluorides using SHC5 and KF, expanding click chemistry with high efficiency and low environmental impact.
This green process, yielding only NaCl and KCl as byproducts, could become the preferred method in chemical and industrial synthesis. The study is published in ACS Sustainable Chemistry & Engineering.
The concept of "click chemistry" is known for its high chemical selectivity, high yield, and rapid connection of molecules. Since its inception, click chemistry has demonstrated broad utility across various fields, including synthesis, materials science, chemical biology, and pharmaceutical development, garnering immense popularity.
However, sulfonyl fluoride is a key compound in the sulfur-fluorine exchange (SuFEx) click reaction, which links molecules together, and its synthesis initially required the use of SO2F2 gas or KHF2, both of which are highly toxic and difficult to handle. To achieve the safe and environmentally-friendly synthesis of sulfonyl fluoride, synthetic chemists have explored various chemical reaction processes.
In this study, sulfonyl fluoride was efficiently synthesized for the first time by reacting the easily handled and highly reactive SHC5 and KF (potassium fluoride) with thiols or disulfides. This is a green synthetic process that produces only non-toxic sodium and potassium salts as by-products, resulting in minimal environmental impact.
This chemical reaction enabled the environmentally friendly and tailor-made synthesis of a broad scope of sulfonyl fluorides containing aromatic, aliphatic, and heterocyclic groups.
The synthetic protocol is very simple, enabling the low-cost, scalable, and safe production of sulfonyl fluorides. This new method is expected to become the first choice for sulfonyl fluoride synthesis in both the chemical and industrial sectors.
"Developing new organic synthetic protocols to create useful compounds, such as pharmaceuticals, is a highly important research theme, particularly from the perspective of the Sustainable Development Goals (SDGs)," write corresponding authors of the study Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem.
"Furthermore, developing reaction processes that consider the environmental impact of by-products is becoming increasingly important. We will continue to disseminate research from Japan that focuses on the green synthesis of useful compounds with ripple effects across various fields."
More information: Sho Yamahara et al, Green and Efficient Protocols for the Synthesis of Sulfonyl Fluorides Using Potassium Fluoride as the Sole Fluorine Source, ACS Sustainable Chemistry & Engineering (2024). DOI: 10.1021/acssuschemeng.4c03951
Journal information: ACS Sustainable Chemistry & Engineering
Provided by Osaka University