This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
SuFEx as a new generation of click chemistry: Synthesis and development of linkers
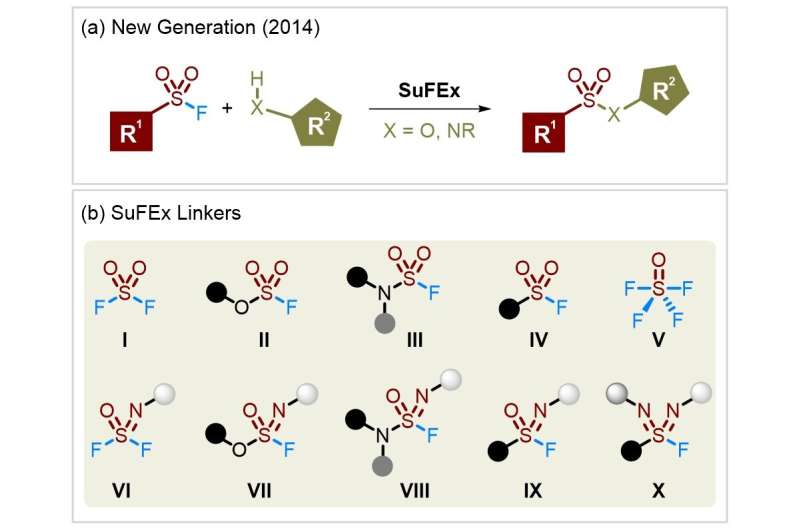
Organosulfur(VI) fluoride compounds display excellent chemical stability, and yet can efficiently realize diverse linkages via the activation of specific conditions. Based on its unique nature, Sharpless, Dong and coworkers introduced SuFEx as a new generation of click reaction in 2014. Since then, SuFEx has become an active research area, frequently occurring in chemical biology, drug discovery, and material chemistry.
Importantly, a selection of SuFEx linkers has been developed, among which FSO2-containing molecules (I-IV) occupy a predominant position. Relative to the corresponding chlorides, S(VI) fluorides are not susceptible to hydrolysis and reduction. The reaction of SO2F2 with available phenols and amines affords fluorosulfates (II) and sulfamoyl fluorides (III) respectively.
Alternatively, solid +SO2F precursors are synthesized with favorable reactivity and chemoselectivity, even accessing the base-sensitive monosubstituted sulfamoyl fluorides. Strategies for the assembly of sulfonyl fluorides (IV) are diverse, which are divided into three bond-breaking modes including S–C bond, S–F bond, and both.
Typical routes mainly rely on the Cl-F exchange, and RSO2Cl substrates are obtained directly or in situ. New reaction models involving the •SO2F species and the insertion of SO2 were used to forge sulfonyl fluorides, thus skirting unpleasant thiols and enabling variation of attached carbon fragments to be unfettered. Additionally, photocatalysis and electrocatalysis are recently deployed for the synthesis of S(VI)-fluoride compounds in a safe and green manner.
The development of FSO2-bearing linkers has considerably spurred its aza-analogs into SuFEx chemistry. Significantly, an additional handle on the nitrogen is offered, which expediently adjusts the property of S(VI) fluorides. SOF4 (V) as a 3-dimensional SuFEx hub combines with primary amines to construct iminosulfur oxydifluorides (VI).
The SOF4-derived difluorides, analogous to SO2F2, can forge the S–O and S–N bonds (VII and VIII) and even extend to the S–C bond (IX) via metal reagents. Other precursors, such as ArS-NPhth and Tr-NSO, are used to access aza-sulfonyl fluorides (IX). The oxidative chlorination-fluorination protocol is still the most common method for sulfonimidoyl fluorides. A notable recent advance is sulfondiimidoyl fluorides (X), which themselves feature two S=N bonds with tremendous potential.
Despite remarkable progress in the assembly of these SuFEx linkers, the established methods still suffer from several limitations: (1) access to heteroatom-linked S(VI)-fluorides depends on poisonous gases, especially SOF4, impeding their widespread application; (2) expensive oxidative fluoride sources frequently occur in the synthesis of sulfonyl fluorides with poor atom efficiency; (3) assembly of sulfonimidoyl fluorides generally originates from sulfur-containing compounds (notably sulfinamides), which enables complex functional molecules to be inaccessible; (4) chiral SuFEx linkers with the S=N fragment are less explored, and only a few examples are disclosed to access enantioenriched sulfonimidoyl fluorides via optically pure substrates.
The researchers believe that the abovementioned hurdles will be addressed in the near future, and safer, more efficient, and modular protocols will be established to forge S(VI)–F linkers, thus facilitating the development and application of SuFEx chemistry.
The findings are published in the journal National Science Review.
More information: Daming Zeng et al, Advances in the construction of diverse SuFEx linkers, National Science Review (2023). DOI: 10.1093/nsr/nwad123
Provided by Science China Press