This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemical and transportation industries could get a boost with new catalyst coating
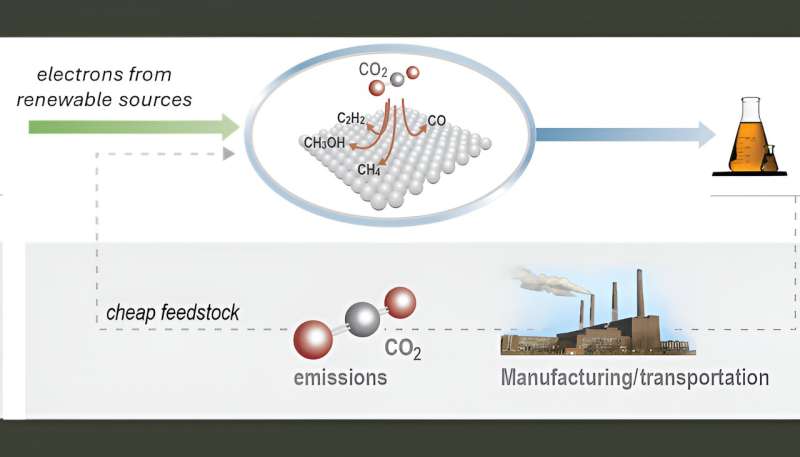
Coupling electrochemical conversion of the greenhouse gas CO2 with renewable electricity sources—such as solar and wind—promises green production of high-demand chemicals and transportation fuels. Carbon dioxide coupling products such as ethylene, ethanol and acetic acid are particularly useful as feedstocks for the chemical industry and powering vehicles.
While designs for efficient and scalable electrolyzers (which use electricity to drive chemical transformations) with industrially relevant current densities have been developed, commercialization efforts have been hindered by the stability and selectivity of the catalysts.
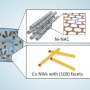
To tackle this challenge, Lawrence Livermore National Laboratory (LLNL) and collaborators have developed a catalyst coating platform that uses physical vapor deposition (PVD), which offers precise control over thickness, composition, morphology and porosity.
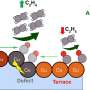
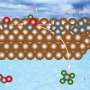
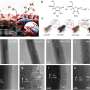
To date, only copper and its alloys have been demonstrated to efficiently convert CO2 into multi-carbon products, such as ethylene, ethanol, acetate and propanol. The challenge in catalyst development is to decouple catalyst performance from catalyst integration-related effects.
This is specifically true when comparing catalysts that have been fabricated and integrated with different methods and are tested in different electrolyzer configurations, or prepared from inks with varying compositions.
"We came up with a new scalable and tunable catalyst platform that allows catalyst composition to be tuned without changing catalyst morphology or catalyst integration in the electrolyzer," said LLNL materials scientist Juergen Biener, a lead author of a paper published in the journal Small. Other LLNL researchers include Zhen Li, Stephen Weitzner, Sneha Akhade and Xie Liu.
The team, which also includes researchers from the University of Delaware, Washington University and the University of Pennsylvania and industry partner Twelve Benefits Corporation, used their PVD platform to systematically explore the performance of copper-based dilute alloy catalysts that are difficult to synthesize and integrate otherwise.
Guided by theory, the team developed several copper-based dilute alloy catalysts that promote coupling of the intermediate carbon monoxide toward the desired multicarbon products.
"The results demonstrate the power of using dilute alloys to systematically tune the energy landscape of CO2 electrolysis to increase the effectiveness of creating cleaner feedstocks for the chemical and transportation industries," said LLNL scientist Joel Varley, who led the simulation efforts of this work.
Beyond providing fine control over coating uniformity, thickness and composition, PVD methods produce less waste and are less labor-intensive than traditional electrodeposition methods, making them more cost effective despite higher capital costs. The development could lead to improvements in the chemical and transportation industries.
More information: Bradie S. Crandall et al, Cu Based Dilute Alloys for Tuning the C2+ Selectivity of Electrochemical CO2 Reduction, Small (2024). DOI: 10.1002/smll.202401656
Journal information: Small
Provided by Lawrence Livermore National Laboratory