This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Investigating the poisoning effect of carbon deposition during CO₂ electroreduction
A research team has proposed new understandings of the poisoning effect of carbon deposition during carbon dioxide reduction reaction (CO2RR) on the active sites of Cu electrode. The study was published in Precision Chemistry.
Copper (Cu)-based catalysts serve as the most efficient electrochemical catalysts for CO2RR. However, their long-term stability at high current densities is unsatisfactory. The reasons for the deactivation of Cu catalysts are complex. It is widely observed that carbon deposition can lead to severe catalyst deactivation. While there are few reports on electrochemical carbon deposition in the field of CO2RR, which hinders the improvement of Cu-based catalysts.
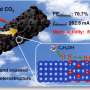
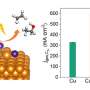
Based on this, the researchers used in-situ spectroscopy techniques and trace analysis techniques to confirm that the production and covering of carbon deposition on polycrystalline Cu foil during CO2RR are the direct causes of catalyst deactivation, clarifying the inherent correlation between carbon deposition and applied potential.
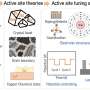
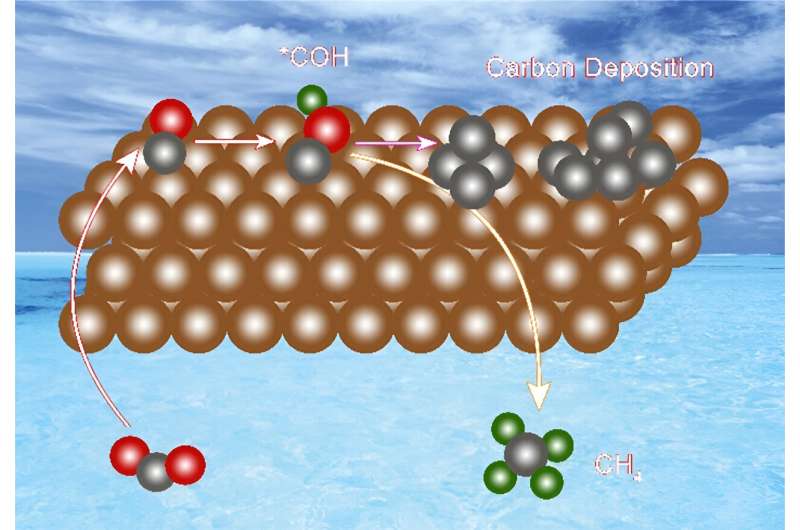
Electrochemical carbon deposition exists in a thin film-like form coating the surface of catalyst particles, with a structure and composition similar to amorphous carbon. This type of carbon had poor conductivity and mass transfer capabilities, hindering the transfer of reaction gases and intermediates during CO2RR.
Based on a series of controlled experimental results, researchers elucidated the mechanism of carbon deposition generation: during CO2RR, the key intermediate *CO combines with protons to form *COH intermediates which further combine with protons and then dehydrate to form adsorbed *C. Adsorbed *C, as a general intermediate, can either proceed with *CH by hydrogenation to generate CH4 or directly desorb to form the deposited carbon.
The research team was led by Professor Gao Minrui from Hefei National Laboratory for Physical Sciences at the Microscale at the University of Science and Technology of China (USTC).
More information: Jing-Wen DuanMu et al, Investigation and Mitigation of Carbon Deposition over Copper Catalyst during Electrochemical CO2 Reduction, Precision Chemistry (2024). DOI: 10.1021/prechem.4c00002
Provided by University of Science and Technology of China