This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Probing carbon capture, atom-by-atom with machine-learning model
A team of scientists at Lawrence Livermore National Laboratory (LLNL) has developed a machine-learning model to gain an atomic-level understanding of CO2 capture in amine-based sorbents. This innovative approach promises to enhance the efficiency of direct air capture (DAC) technologies, which are crucial for reducing the excessive amounts of CO2 already present in the atmosphere.
Despite ongoing efforts to decarbonize the economy, the U.S. Department of Energy projects that the majority of national energy production will still come from non-renewable sources by 2050. This underscores the urgent need to not only develop new renewable energy technologies but also to improve methods for capturing and storing CO2 emissions.
Amine-based sorbents have emerged as a promising solution, efficiently binding CO2 even at ultra-dilute conditions. The low cost of these sorbents has enabled several companies to scale up this technology, demonstrating DAC as a feasible way to combat global warming. However, significant knowledge gaps remain in the chemistry of CO2 capture under experimentally relevant conditions.
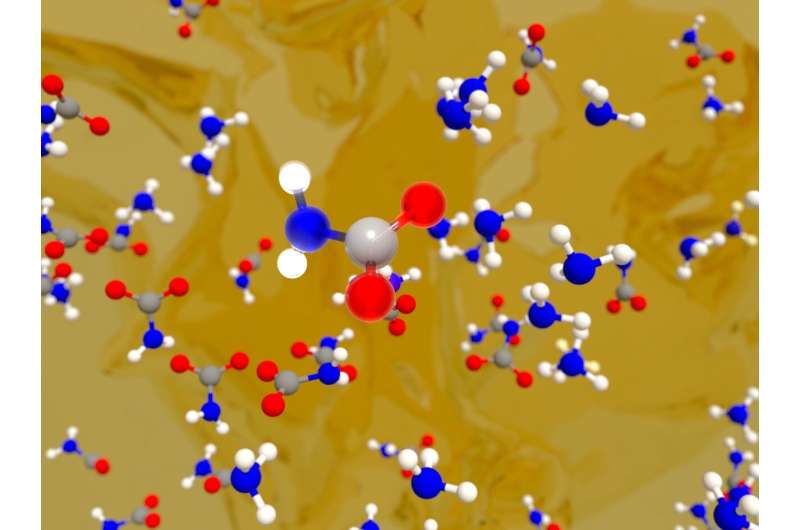
The LLNL team's machine learning model has revealed that CO2 capture by amines involves the formation of a carbon-nitrogen chemical bond between the amino group and CO2, alongside a complex set of solvent-mediated proton transfer reactions. These proton transfer reactions are critical for the formation of the most stable CO2-bound species and are significantly influenced by quantum fluctuations of protons.
"Our method can be extended to amines with different chemical compositions, highlighting the impact of machine learning in understanding the fundamental chemistry involved in CO2 capture under realistic conditions," said Marcos Calegari Andrade, lead author of a paper appearing in Chemical Science.
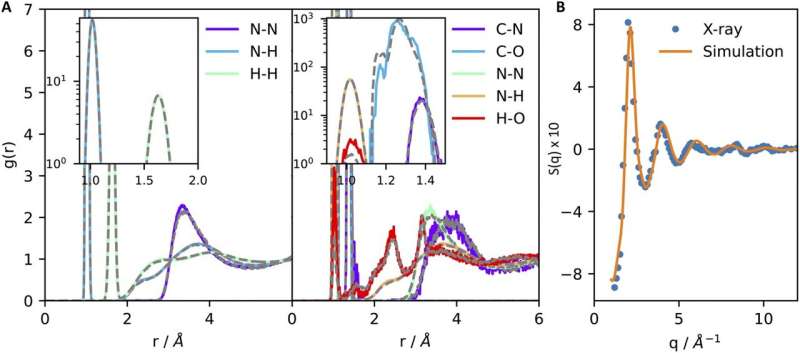
Using a combination of grand-canonical Monte Carlo and enhanced sampling methods in molecular dynamics, the researchers obtained quantities directly accessible by experiments.. These results provide a vital connection to laboratory measurements and pave the way for a future feedback loop between simulations and experiments.
"By integrating machine learning with advanced simulation techniques, we've created a powerful approach that bridges theoretical predictions and experimental validations of CO2-capture mechanisms in a way not accessible by traditional simulation techniques," said LLNL scientist Sichi Li, co-corresponding author and project theory lead.
"This research not only advances our understanding of CO2 capture mechanisms but also provides a new and critical tool for designing next-generation materials that can contribute to net-zero greenhouse gas emissions", said Simon Pang, co-corresponding author and project principal investigator.
LLNL co-authors also include Tuan Anh Pham and Sneha Akhade.
More information: Marcos F. Calegari Andrade et al, Machine learning demonstrates the impact of proton transfer and solvent dynamics on CO2 capture in liquid ammonia, Chemical Science (2024). DOI: 10.1039/D4SC00105B
Journal information: Chemical Science
Provided by Lawrence Livermore National Laboratory