This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Depolymerization method achieves exclusive chemical recycling of PET from cloth waste and plastic waste mixtures

A research team led by Professor Kotohiro Nomura from Tokyo Metropolitan University has developed a method for the depolymerization of PET (polyethylene terephthalate) using alcohols and an inexpensive, readily available iron trichloride catalyst. This method can be applied to the selective chemical recycling of both textile and plastic waste mixtures.
The research is published in the journal Industrial Chemistry & Materials.
Plastic waste is a significant environmental issue that requires urgent attention. However, the rate of plastic reuse (material recycling) remains low, particularly in the case of chemical recycling into raw materials, a process known as chemical recycling. Polyesters, which consist of repeated "ester bonds" formed by the reaction of carboxylic acid and alcohol, are commonly used in plastic bottles and clothing.
If these ester bonds could be completely broken, polyester could be reverted to its raw materials. Conventional methods, however, necessitate high temperatures and large amounts of acidic or basic substances.
Therefore, a simple, cost-effective, and environmentally friendly method is highly sought after. Additionally, there is a global demand for the development of selective depolymerization of polyester from plastic waste, especially from textile waste, which is a mixture of polyester and cotton.

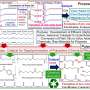
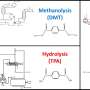
The research team has now developed a method for acid- and base-free depolymerization of PET bottles using ethanol and either FeCl3 or FeBr3, yielding diethyl terephthalate (DET) and ethylene glycol (EG) with high selectivity (98–99%). Iron trichloride (FeCl3), which is inexpensive and widely available, demonstrated superior catalytic performance at 160–180ºC, comparable to their previous results using titanium catalysts.
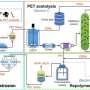
Notably, this method enabled the exclusive and selective depolymerization of PET from textile waste, which comprises PET and a mixture of PET and cotton, yielding DET and EG while quantitatively recovering cotton waste. The catalyst also facilitated the selective depolymerization of PET from plastic waste, including polyethylene.
The development of a straightforward method for the exclusive and selective depolymerization of PET is particularly desired for the chemical recycling of textile waste. This method of exclusive chemical recycling of PET from plastic wastes offers a promising solution for achieving a circular economy.
More information: Nor Wahida Binti Awang et al, Depolymerization of PET with ethanol by homogeneous iron catalysts applied for exclusive chemical recycling of cloth waste, Industrial Chemistry & Materials (2024). DOI: 10.1039/D4IM00081A
Provided by Tokyo Metropolitan University