This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemical production gets a cleaner boost from a new electrochemical method
A new electrochemical method can make chemical production cleaner and more energy-efficient.
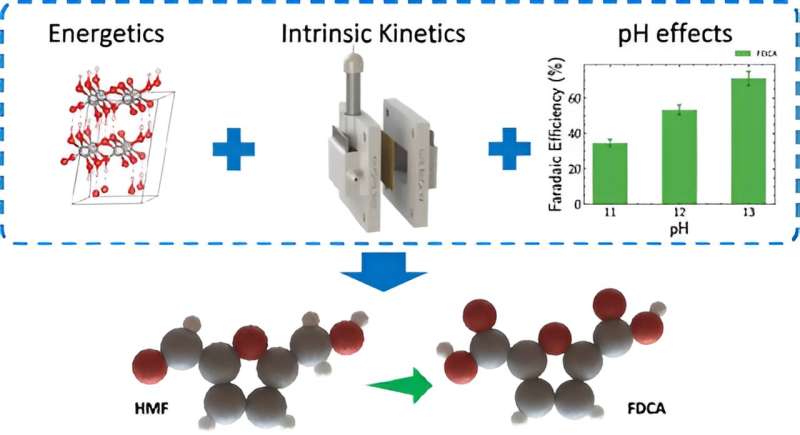
Using thin film nickel anodes, a team of Lawrence Livermore National Laboratory (LLNL) scientists and collaborators have figured out how to clean up chemical production.
When studying a new electrochemical reaction, using thin films is important because they give a consistent surface to work with, making it easier to understand how the catalyst actually works.
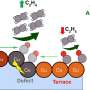
"Thin films eliminate complications from porous/rough structures or varying thicknesses, allowing us to focus on the true properties of the catalyst material," said LLNL postdoc Aditya Prajapati, first author of a paper appearing in the journal ACS Catalysis.
A corresponding author and PI of this project, Christopher Hahn, said, "This helps us better understand the chemical reactions and how to improve them, leading to more efficient processes."
Current electrolyzers (which convert electrical energy into molecules with high potential-energy densities) struggle with energy efficiency due to the oxygen evolution at the anode. The team found that replacing this oxygen evolution reaction with biomass oxidation could cut energy use by more than 50%, addressing both efficiency and the need for sustainable biomass conversion.
This process cleans up chemical production by converting 5-Hydroxymethylfurfural (HMF), derived from biomass, into 2,5-Furandicarboxylic acid (FDCA), a valuable building block for sustainable plastics like PEF (a bio-based polymer). The team concluded that the process reduces petroleum dependency and carbon emissions.
"Unlike traditional methods that require high temperatures and produce toxic waste, our electrochemical method is cleaner and more energy-efficient," said Nitish Govindarajan, one of the corresponding authors of this work.
By utilizing biomass as the starting material, the process supports the use of renewable feedstock, promoting a circular economy and reducing reliance on finite fossil fuel reserves.
Electrochemical oxidation of HMF to FDCA consumes less energy compared to traditional methods, contributing to lower carbon footprints in chemical production. Additionally, it also has the potential to have a zero-carbon footprint if paired with renewable electricity.
Other LLNL authors include Wenyu Sun, Jeremy Feaster and Sneha Akhade as well as researchers from Université de Montréal and the University of Bonn.
More information: Aditya Prajapati et al, Mechanistic Insights into the Electrochemical Oxidation of 5-Hydroxymethylfurfural on a Thin-Film Ni Anode, ACS Catalysis (2024). DOI: 10.1021/acscatal.4c01448
Journal information: ACS Catalysis
Provided by Lawrence Livermore National Laboratory