This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Integration of theory prediction and experimental electrooxidation of glycerol on nanosheets
Glycerol, a major by-product of biomass refining accounting for approximately 10% of the yield, presents a significant challenge due to its global surplus. The presence of multiple active hydroxyl groups in glycerol unveils vast potential for the production of high-value chemicals. Formic acid (FA), a key product of glycerol conversion, is a critical organic chemical raw material with high demand in sectors such as pesticides, pharmaceuticals, and energy.
Oxidizing glycerol to FA not only mitigates the waste caused by resource surplus but also caters to the future needs of FA fuel cells. Currently, the industrial production of FA primarily relies on methanol derived from petroleum and natural gas, making the electrocatalytic conversion of biomass-based glycerol into FA highly promising.
However, the electrocatalytic oxidation of glycerol reaction (RGOR) is complex, involving dehydrogenation, adsorption/desorption, and C-C bond breaking of reaction intermediates, posing challenges to the reaction's efficiency and selectivity.
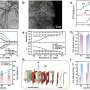
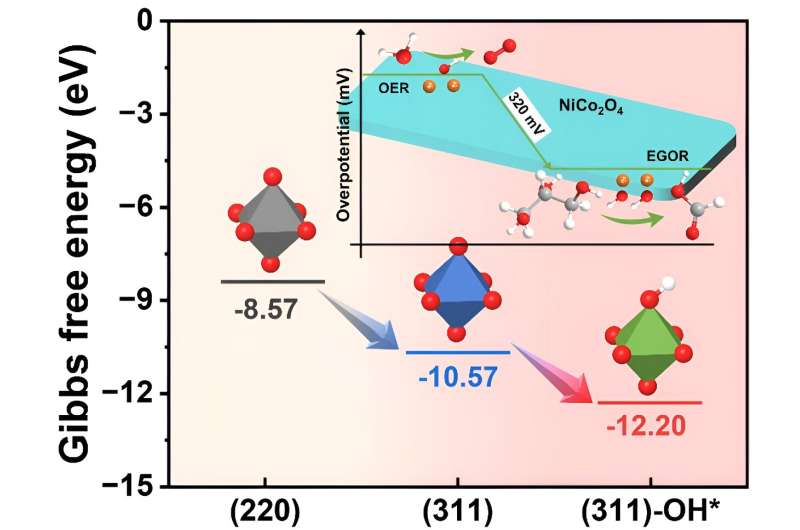
Recently, a research team led by Prof. Kai Yan of Sun Yat-sen University, China, utilizing a combination of density functional theory (DFT) calculations and experimental methods, has unveiled the crucial role of the active species OH* in the EGOR process in producing FA. DFT analysis, starting from a thermodynamic perspective, investigated the mechanism of OH* in the EGOR process.
It was found that surface OH* species, by reducing the adsorption energy of glycerol on the NiCo2O4 catalyst surface (from -12.20 to -10.57 eV), facilitated the EGOR process and optimized the rate-determining step (RDS) by altering the adsorption energy of intermediates, shifting from the less efficient dehydrogenation of glyceric acid to the more efficient dehydrogenation step of glyceraldehyde.
Moreover, the adsorption energy of OH* during the EGOR process was significantly lower compared to the oxygen evolution reaction (OER) process (0.66 vs. 2.70 eV), indicating the preferential occurrence of EGOR over OER.
Further, the performance of a meticulously designed NiCo2O4 electrode in EGOR was investigated through electrochemical methods. In a mixed electrolyte of 1 mol L-1 KOH and 0.1 mol L-1 glycerol, the onset potential of the electrode dropped to 1.16 VRHE, significantly outperforming OER. Rotating ring-disk electrode (RRDE) experiments also confirmed the preferential occurrence of EGOR, aligning with the DFT analysis findings.
Based on the conventional proton-coupled electron transfer mechanism, two possible electrochemical oxidation pathways (direct oxidation pathway and indirect oxidation pathway) of OH* have been investigated by using multi-step potentiation and simultaneous electron resonance methods. Experimental results demonstrated that the exceptional performance of the NiCo2O4 electrode in EGOR is closely related to the in-situ generation of OH* directly participating in the reaction.
In a long-term cycle stability test of 120 h, the catalyst also showed an efficient and stable glycerol conversion rate (89%) and formic acid selectivity (70%). This work provides valuable guidance and insights for the design and development of efficient and stable catalysts for glycerol oxidation. The results were published in Chinese Journal of Catalysis.
More information: Yan Duan et al, Integration of theory prediction and experimental electrooxidation of glycerol on NiCo2O4 nanosheets, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64585-1
Provided by Chinese Academy of Sciences