This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New approach developed for electrocatalytic H₂O₂ production and biomass upgrading
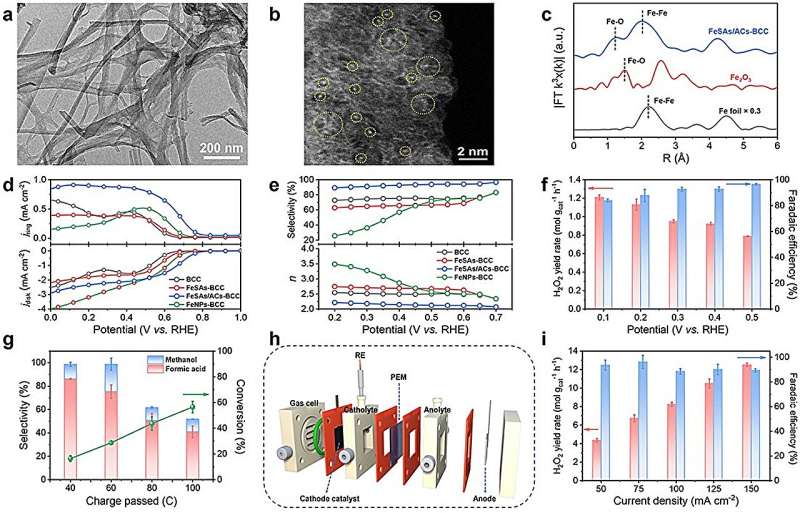
Scientists from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences have synthesized an oxygen-coordinated Fe single atom and atom cluster catalyst that exhibits superior electrocatalytic performance for hydrogen peroxide (H2O2) production and biomass upgrading. The research is published in Angewandte Chemie International Edition.
H2O2 is a widely used chemical with applications in diverse fields such as environment, energy, and health care. While traditionally produced through energy-intensive processes, electrocatalytic synthesis offers a more environmentally friendly and efficient method using water and oxygen.
However, this approach requires advanced electrocatalysts for high-yield and selective H2O2 production, and further attention is needed to utilize the generated H2O2, particularly in electrochemical organic oxidation processes. This offers significant potential for value-added applications beyond environmental remediation.
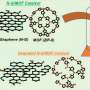
For this study, the researchers used bacterial cellulose as an adsorption regulator and carbon source in combination with a multi-step approach involving wet chemical impregnation, pyrolysis, and acid etching processes to create a catalyst termed FeSAs/ACs-bacterial cellulose-derived carbon (BCC), consisting of oxygen-coordinated Fe single atoms (SAs) and atom clusters (ACs).
The presence of both Fe SAs and clusters was confirmed using advanced imaging techniques such as aberration-corrected scanning transmission electron microscopy. The atomic structure of Fe was also determined by X-ray fine structure absorption spectroscopy and X-ray photoelectron spectroscopy.
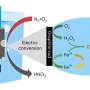
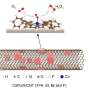
This catalyst showed excellent electrocatalytic performance and selectivity for the 2-electron oxygen reduction reaction (2e– ORR) under alkaline conditions. Further H-cell experiments confirmed the accumulation of H2O2 in the electrolyte.
The researchers coupled the in situ generated H2O2 with the electro-Fenton process using ethylene glycol as the reactant and acidified 0.1 M Na2SO4 as the electrolyte. This resulted in a high rate of ethylene glycol conversion and high selectivity for formic acid, demonstrating that the electro-Fenton process has the potential to improve biomass-derived feedstocks through oxidative upgrading.
They also developed a three-phase flow cell based on the gas diffusion electrode to further improve the H2O2 yield.
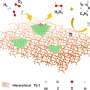
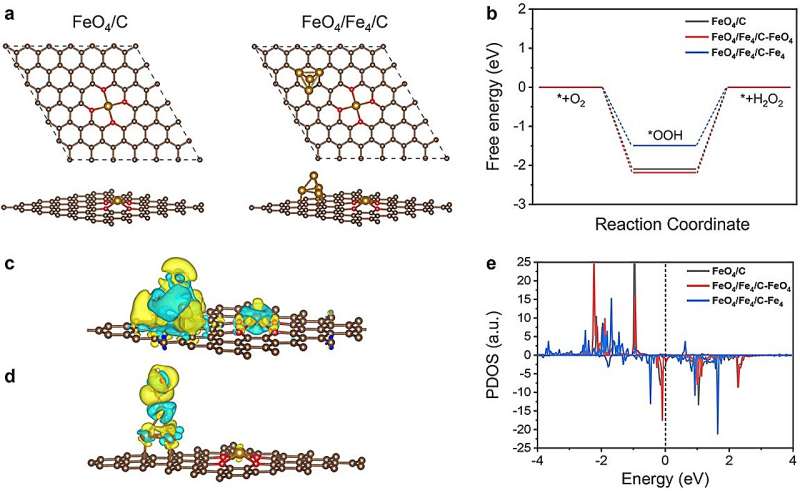
Density functional theory analyses indicated that the actual catalytically active sites in the 2e– ORR process were the Fe clusters, and the electronic interaction between Fe single atoms and Fe clusters could significantly enhance the electrocatalytic performance toward 2e– ORR.
This work will be helpful for the design and development of atomic-level electrocatalysts for high-efficiency 2e– ORR to H2O2 and biomass upgrading.
More information: Hui Xu et al, Atomically Dispersed Iron Regulating Electronic Structure of Iron Atom Clusters for Electrocatalytic H2O2 Production and Biomass Upgrading, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202314414
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences