Researchers realize efficient hydrogen-peroxide production in acid
As one of the 100 most important chemicals in the world, hydrogen peroxide (H2O2) is mainly produced by the energy- and waste-intensive anthraquinone oxidation (AO) method. Replacing the AO method with a more environmentally-benign electrochemical two-electron oxygen reduction reaction (2e- ORR) depends on cheap and efficient catalysts.
However, metal-free, carbon-based catalyst as a promising candidate behaves encouragingly only under neutral or alkaline conditions, where H2O2 is unstable for collection or unfavorable for linking applications, i.e., the e-Fenton reaction. Moreover, it remains challenging to identify the real active catalytic sites and the underlying 2e- ORR mechanism.
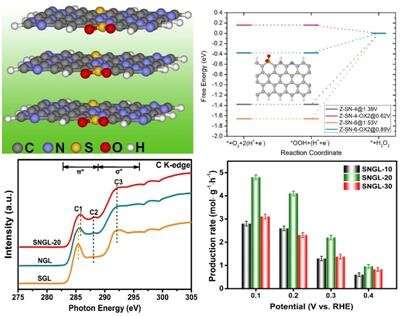
In a study published in Chem Catalysis, a research group led by Prof. Guan Lunhui from the Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences developed a metal-free, highly efficient acidic 2e- ORR catalyst with recorded hydrogen peroxide production rate based on pyrimidine-assisted active site modulation and S, N-codoped few-layered graphene for valence electronic optimization.
The catalyst exhibited exceptional activity and selectivity for 2e- ORR in acid. The H2O2 selectivity reaches 90%~100% over a potential range of 0.20~0.55 V and the maximum H2O2 production rate (4.8 mol·g-1·h-1) exceeds all the reported H2O2 production performance for carbon material-based catalysts.
Experiments and density-functional-theory simulations (contributed by Prof. Chai Guoliang) revealed that the synergy effect of the combined oxidized sulfur and pyridinic-N functional motif can lower the Fermi level of valence electronic states of active edge carbon sites, and thus leads to suitable binding strength of *OOH intermediate for high selectivity and performance 2e- ORR for H2O2 formation.
In particular, the researchers observed an obvious peak-shift to high energy of C 1s excitation in near edge X-ray absorption fine structure with S incorporation, as solid evidence for the valence electronic optimization of carbon catalyst surface.
In addition, coupled with Fenton reaction for an electron-Fenton process, it can degrade a model organic pollutant (methylene blue [MB], 50 ppm) to colorless in a short time of 15 min.
This study not only creates an efficient carbon-based catalyst for H2O2 production in acid, but also provides a useful electronic property optimization route for future tuning of carbon-based materials catalysts.
More information: Jiaoxing Xu et al, Pyrimidine-assisted synthesis of S, N-codoped few-layered graphene for highly efficient hydrogen peroxide production in acid, Chem Catalysis (2022). DOI: 10.1016/j.checat.2022.04.011
Provided by Chinese Academy of Sciences