This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Metal-based electrocatalysts for ammonia electro-oxidation reaction to nitrate/nitrite: Past, present and future
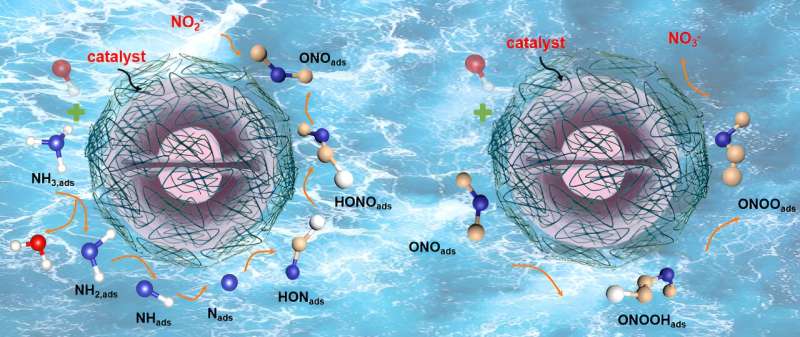
Recently, a research team led by Prof. Ji Liang from Tianjin University, China, systematically introduced the research progress on the preparation of nitrate/nitrite by ammonia electro-oxidation reaction and proposed different strategies to enhance the electrocatalytic performance of the catalysts by modulating their composition and structure to inhibit the side reactions and electrode corrosion in the electrocatalytic process, and finally proposes the opportunities and challenges faced by ammonia electrocatalysis as well as its development trend.
The results were published in Chinese Journal of Catalysis.
Nitrite and nitrate (NO2/3−) are important substances in industry, agriculture, and food engineering. The current process of manufacturing NO2/3− by Ostwald oxidation is often accompanied by significant energy consumption and greenhouse gas emissions.
Electrocatalytic oxidation of ammonia is a low-emission and energy-efficient low-temperature process that allows for the continuous production of NO2/3−, avoiding the formation of harmful N2O, and can be fully powered by renewable electricity. Most of the current studies on the ammonia oxidation reaction have focused on hydrogen production from ammonia cracking and direct ammonia fuel cells, while the study of the relevance of ammonia conversion to NO2/3− has received little attention.
Therefore, based on summarizing the work in recent years, we provide an overview of the reaction mechanism and design ideas of catalysts to provide theoretical guidance for researchers to develop catalysts focusing on NO2/3− generation and to lay the foundation for future nitrogen cycle systems.
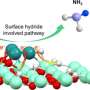
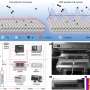
Based on the possible reaction mechanism of electrocatalytic ammonia oxidation reaction (AOR), this paper introduces the reaction conditions, detection methods, in situ characterization methods, and theoretical calculations of AOR. Based on the summary of the factors affecting the AOR catalysts, this paper proposes the design strategies and synthesis methods of electrocatalysts in recent years and gives an outlook on the future development of the ammonia field.
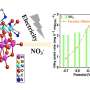
Firstly, the key difficulties of AOR are discussed based on the reaction principle and the adsorption path of reaction intermediates. Then, the testing requirements of AOR and the important roles of in-situ characterization in the mechanistic study of AOR are systematically summarized, and the important roles of density-functional theory (DFT) for the study of reaction energy barriers and catalyst electron-orbital distributions in the catalytic process of AOR are discussed.
In addition, controllable strategies such as catalyst alloy design, interface engineering, amorphization treatment, and mono- or diatomic modulation can help to inhibit the side reactions as well as the damage to the electrodes by the corrosive substances generated during the electrolysis process.
Finally, the progress of ammonia oxidation in photo-, thermo-, and biocatalytic applications is presented, and the current challenges and solution strategies for AOR, such as the combination of advanced material design with theoretical calculations, are suggested to help find new high-performance AOR electrocatalysts.
The improvement of the catalytic system and optimization of the reactor will accelerate the industrialization of large-scale green, efficient, and low-energy electrocatalytic preparation of NO2/3−.
In summary, the progress made in the field of AOR is sufficient to confirm the feasibility of ammonia electrooxidation for the preparation of NO2/3− for industrial production, which brings new opportunities for the growing demand of NO2/3− supply.
Although AOR still faces the problems of low performance and immature process, with the combination of theoretical and experimental research and the development and utilization of in-situ characterization technology, efficient and stable AOR catalysts will appear in the future, and the carbon-free energy recovery network system centered on AOR will be established rapidly.
More information: Yunrui Tian et al, Metal-based electrocatalysts for ammonia electro-oxidation reaction to nitrate/nitrite: Past, present, and future, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64576-0
Provided by Chinese Academy of Sciences