This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Exploring developments and challenges in electrochemical nitrate reduction for ammonia synthesis
Ammonia is a necessary feedstock to produce nitrogen-based fertilizers, chemicals, pharmaceuticals, and polymers. To date, about 80% of global ammonia is used to produce nitrogen-based fertilizers which relates to 50% of global food production.
The global production of ammonia is about 180 million metric tons per year through the carbon-intensive and highly energy-consuming Haber-Bosch process. The high energy consumption, high carbon intensity, and high capital investment of the Haber-Bosch process make the development of environmentally sustainable and affordable routes for ammonia synthesis under ambient conditions more urgent.
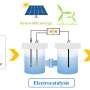
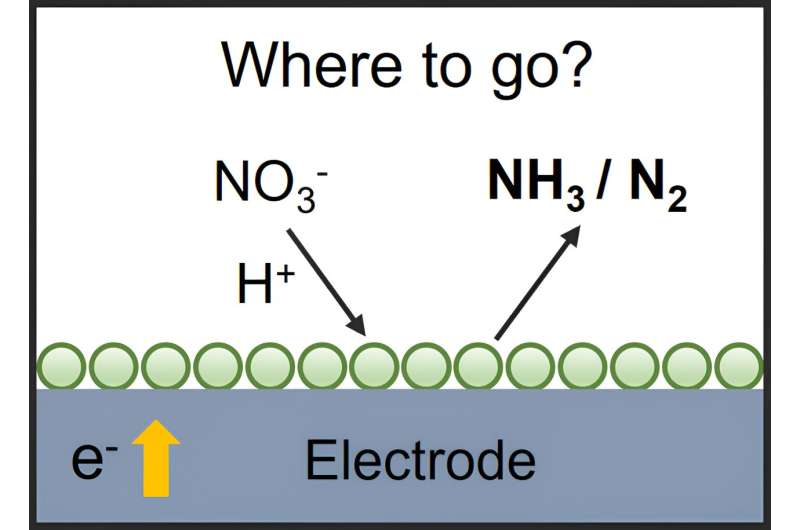
The electrochemical ammonia synthesis, powered by renewable energy, offers a promising approach to this puzzle. Electrocatalytic nitrate reduction reaction (NtrRR) for ammonia synthesis at ambient conditions has the potential to address environmental issues and obtain useful chemicals simultaneously.
Although many efforts have been made to develop an efficient catalyst for improving ammonia Faraday efficiency and current density in the NtrRR, it still faces some scientific and practical challenges. This perspective aims to discuss those challenges and propose corresponding solutions.
In a recent perspective from the Department of Physics at the Technical University of Denmark, Dr. Xianbiao Fu explores the promising developments and challenges in electrochemical nitrate reduction for ammonia synthesis. This perspective was published in the Chinese Journal of Catalysis.
Dr. Fu emphasizes the importance of sustainable nitrate supply, proposing three potential routes: utilizing industrial wastewater, employing plasma processes, and exploring electrochemical oxidation of nitrogen. Sustainable and affordable nitrate production is identified as a key challenge for the viability of electrochemical nitrate reduction.
The study underscores the significance of understanding the species balance in nitrate reduction reactions, especially regarding changes in electrolyte pH over time. This factor, often neglected, could impact the long-term stability and performance of the electrochemical system.
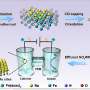
One notable challenge highlighted is the mass transfer limitation of nitrate, which requires innovative solutions for enhancing the transport of nitrate at high current densities. This perspective discusses potential strategies, such as microfluidic reactors, stirring, and the use of high-concentration nitrate electrolytes.
The stability of catalysts for nitrate reduction is identified as a crucial aspect, especially for industrial applications. While certain transition metals like copper and cobalt have shown high activity and selectivity, their stability over prolonged use remains a concern. Strategies such as alloying, encapsulation, and immobilization are proposed to improve catalyst stability.
In conclusion, this perspective provides a comprehensive overview of the opportunities and challenges in electrochemical nitrate reduction for ammonia synthesis.
Fu's insights pave the way for future research directions, emphasizing the need for sustainable nitrate supply routes, understanding species balance, overcoming mass transfer limitations, and ensuring catalyst stability to advance the field towards environmentally friendly and economically viable ammonia synthesis.
More information: Xianbiao Fu, Some thoughts about the electrochemical nitrate reduction reaction, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(23)64521-8
Provided by Chinese Academy of Sciences