This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Electromagnetic field-assisted thermal catalysis enabling low-temperature, low-pressure, large-scale ammonia synthesis
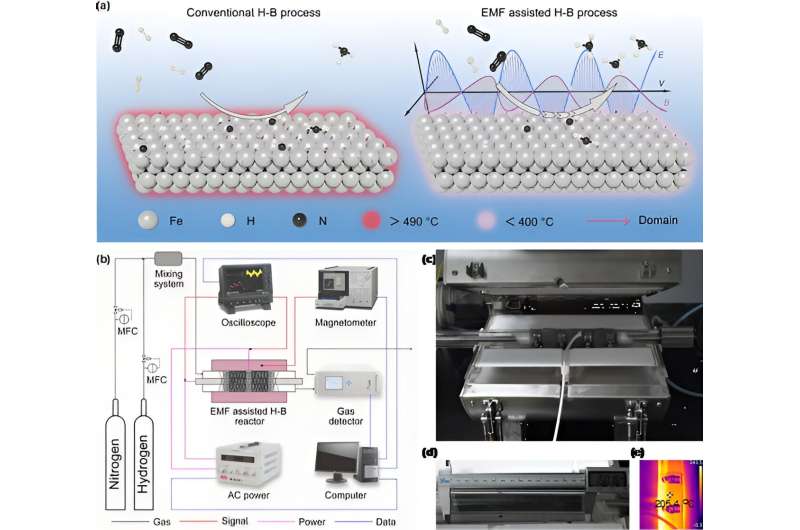
Ammonia (NH3), as one of the most common industrial chemicals, is essential for nitrogenous fertilizer production and shows potential as a next-generation green fuel. Industrial ammonia synthesis relies on the reaction of fossil fuel-derived hydrogen and nitrogen (Haber-Bosch method) under high temperature (~500 °C) and high pressure (>15 MPa), which consumes ∼2% of global power and discharges ∼1.5% of global greenhouse gas.
A team from Tianjin University in China developed an electromagnetic field (EMF)-assisted H-B technique for ammonia synthesis under mild conditions by adopting commercial iron-based catalysts. The onset temperature with EMF assistance is 100 °C, which is obviously lower than that without EMF assistance (300 °C).
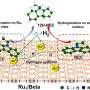
In a typical scale-up experiment with 80 g commercial catalysts, the EMF-assisted H-B technique obviously increases the ammonia yield (~5 times) and decreases the energy consumption (~2.7 times). The enhanced catalytic performance can be ascribed to the EMF inducing more electron transfer from Fe orbitals to N≡N orbitals in both side-on and end-on adsorption modes.
-
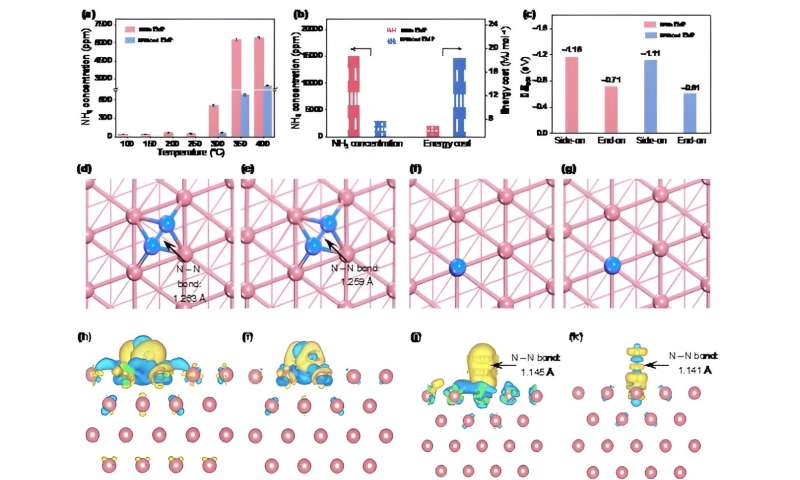
(a) Ammonia concentration under different temperatures with and without an EMF at 1 MPa. (b) Comparison of ammonia concentration and energy cost for the scale-up experiment at 200 ℃ and 1 MPa with and without the EMF. (c) Adsorption energy of N2 on Fe(110) with and without an EMF (1 V Å-1). Atomic structure of N2 side-on adsorption on the Fe(110) surface with (d) and without (e) an external electric field and N2 end-on adsorption on the Fe(110) surface with (f) and without (g) an external electric field. Gray ball: Fe, blue ball: N. Charge density difference of N2 side-on adsorption with (h)and without (i) an external electric field and N2 end-on adsorption with (j) and without (k) an external electric field. The isosurface value is 0.003 e Å-1The yellow isosurface represents electron accumulation, and cyan denotes electron depletion. Credit: ©Science China Press -

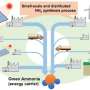
A pilot-scale system includes alkaline water electrolysis equipment, air PSA separation equipment and EMF-assisted H-B equipment. Credit: Science China Press
They constructed a pilot-scale system with a production capacity of 10,000 kg per year for EMF-assisted thermal catalysis at Tianjin University, taking the first step in the development of EMF-assisted thermal catalysis for industrialization from the laboratory.
They are carrying out further scale-up research on EMF-assisted thermal catalysis in Qinghai Province, which is rich in renewable energy, to explore a feasible industrial path for the breakthrough of large-scale storage and transportation bottlenecks of renewable energy to promote the early realization of the "dual carbon" goal.
The research is published in the journal Science Bulletin.
More information: Bao Shun Zhang et al, Electromagnetic field-assisted low-temperature ammonia synthesis, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.07.037
Provided by Science China Press