This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Smells may prime our gut to fight off infection
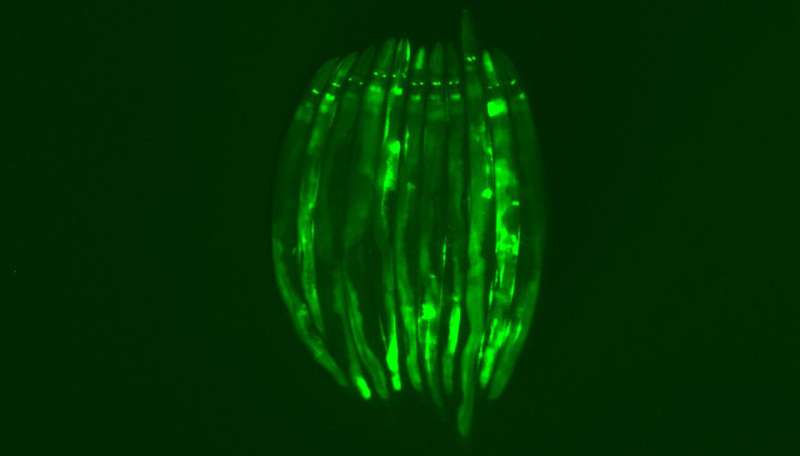
Many organisms react to the smell of deadly pathogens by reflexively avoiding them. But a recent study from the University of California, Berkeley, shows that the nematode C. elegans also reacts to the odor of pathogenic bacteria by preparing its intestinal cells to withstand a potential onslaught.
As with humans, nematodes' guts are a common target of disease-causing bacteria. The nematode reacts by destroying iron-containing organelles called mitochondria, which produce a cell's energy, to protect this critical element from iron-stealing bacteria. Iron is a key catalyst in many enzymatic reactions in cells—in particular, the generation of the body's energy currency, ATP (adenosine triphophate).
The presence in C. elegans of this protective response to odors produced by microbes suggests that the intestinal cells of other organisms, including mammals, may also retain the ability to respond protectively to the smell of pathogens, said the study's senior author, Andrew Dillin, UC Berkeley professor of molecular and cell biology and a Howard Hughes Medical Institute (HHMI) investigator.
"Is there actually a smell coming off of pathogens that we can pick up on and help us fight off an infection?" he said. "We've been trying to show this in mice. If we can actually figure out that humans smell a pathogen and subsequently protect themselves, you can envision down the road something like a pathogen-protecting perfume."
So far, however, there's only evidence of this response in C. elegans. Nevertheless, the new finding is a surprise, considering that the nematode is one of the most thoroughly studied organisms in the laboratory. Biologists have counted and tracked every cell in the organism from embryo to death.
"The novelty is that C. elegans is getting ready for a pathogen before it even meets the pathogen," said Julian Dishart, who recently received his UC Berkeley Ph.D. and is the first author of the study. "There's also evidence that there's probably a lot more going on in addition to this mitochondrial response, that there might be more of a generalized immune response just by smelling bacterial odors. Because olfaction is conserved in animals, in terms of regulating physiology and metabolism, I think it's totally possible that smell is doing something similar in mammals as it's doing in C. elegans."
The work was published June 21 in the journal Science Advances.
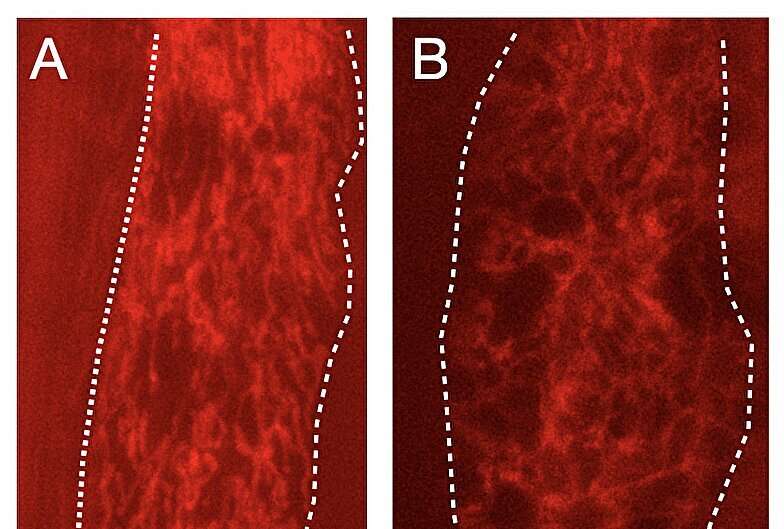
Mitochondria communicate with one another
Dillin is a pioneer in studying how stress in the nervous system triggers protective responses in cells—in particular, the activation of a suite of genes that stabilize proteins made in the endoplasmic reticulum. This activation, the so-called unfolded protein response (UPR), is "like a first aid kit for the mitochondria," he said.
Mitochondria are not only the powerhouses of the cell, burning nutrients for energy, but also play a key role in signaling, cell death and growth.
Dillin has shown that errors in the UPR network can lead to disease and aging, and that mitochondrial stress in one cell is communicated to the mitochondria of cells throughout the body.
One key piece of the puzzle was missing, however. If the nervous system can communicate stress through a network of neurons to the cells doing the day-to-day work of protein building and metabolism, what in the environment triggers the nervous system?
"Our nervous system evolved to pick up on cues from the environment and create homeostasis for the entire organism," Dillin said. "Julian actually figured out that smell neurons are picking up environmental cues and which types of odorants from the pathogens turn on this response."
Previous work in Dillin's lab showed the importance of smell in mammalian metabolism. When mice are deprived of smell, he found, they gained less weight while eating the same amount of food as normal mice. Dillin and Dishart suspect that the smell of food may trigger a protective response, like the response to pathogens, in order to prepare the gut for the damaging effects of ingesting foreign substances and converting that food to fuel.
"Surviving infections was the most important thing we did evolutionarily," Dillin said. "And the most risky and taxing thing we do every single day is eat, because pathogens are going to be in our food."
"When you eat food, it's also incredibly stressful, because the body is metabolizing the food but also generating ATP in the mitochondria from the nutrients that they're incorporating. And that generation of ATP causes a by-product called reactive oxygen species, which is very damaging to cells," Dishart said. "Cells have to deal with this increased existence of reactive oxygen species. So perhaps smelling food can prepare us to deal with that enhanced reactive oxygen species load."
Dillin speculates further that mitochondria's sensitivity to the smell of pathogenic bacteria may be a holdover from an era when mitochondria were free-living bacteria, before they were incorporated into other cells as power plants to become eukaryotes some 2 billion years ago. Eukaryotes eventually evolved into multicellular organisms with differentiated organs—so-called metazoans, like animals and humans.
"There's a lot of evidence that bacteria sense their environment in some way, though it's not always clear how they do it. These mitochondria have retained one aspect of that after being subsumed into metazoans," he said.
In his experiments with C. elegans, Dishart found that the smell of pathogens triggers an inhibitory response, which unleashes a signal to the rest of the body. This became clear when he ablated olfactory neurons in the worm and found that all peripheral cells, but primarily intestinal cells, showed the stress response typical of mitochondria that are being threatened. This study and others also showed that serotonin is a key neurotransmitter communicating this information throughout the body.
Dillin and his lab colleagues are tracking the neural circuits that lead from smell neurons to peripheral cells and the neurotransmitters involved along the way. And he's looking for a similar response in mice.
"I always hate it when I get sick. I'm like, 'Body, why didn't you prepare for this better?' It seems really stupid that you turn on response mechanisms only once you're infected," Dillin said. "If there are earlier detection mechanisms to increase our chances of survival, I think that's a huge evolutionary win. And if we could harness that biomedically, that would be pretty wild."
Other UC Berkeley authors of the paper are Corinne Pender, Koning Shen, Hanlin Zhang, Megan Ly and Madison Webb.
More information: Julian G. Dishart et al, Olfaction regulates peripheral mitophagy and mitochondrial function, Science Advances (2024). DOI: 10.1126/sciadv.adn0014
Journal information: Science Advances
Provided by University of California - Berkeley