This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Crystalline solid could function as a hydrogen carrier by adsorbing and releasing ammonia
All around the world, scientists are striving towards next-generation energy technologies that can help us move away from fossil fuels. Using hydrogen as an energy carrier and clean energy source is perhaps one of the most promising solutions on the horizon.
However, there is a major challenge to overcome before hydrogen economies become a reality: Hydrogen gas is remarkably difficult to store and transport safely, which severely limits its applicability across many fields.
Against this backdrop, a research team from Tokyo Institute of Technology, Japan, and Tokyo University of Science, Japan has been working hard to reach an alternative solution to the hydrogen storage problem. Led by Associate Professor Kosuke Ono, they recently developed a novel compound—simply called 1a—that can adsorb at high density and desorb ammonia (NH3) repeatedly, making it easy to recover ammonia.
This gas is much more convenient to move around and can provide chemical energy just like hydrogen. Their findings have been published in the Journal of the American Chemical Society.
Compared to hydrogen, NH3 does not require cold storage or extremely high pressure, which already saves a lot of energy. Moreover, existing industrial NH3 infrastructure could be readily repurposed for emerging NH3 applications as an energy carrier.
These are but a few of the advantages of NH3. Ono explains, "NH3 is not only a source of hydrogen but also considered a carbon-free energy carrier that produces N2 and H2O upon combustion without producing CO2. Thus, the capture and recovery of NH3 are highly desirable, both from an environmental perspective and with respect to efficient resource use."
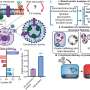
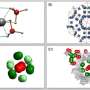
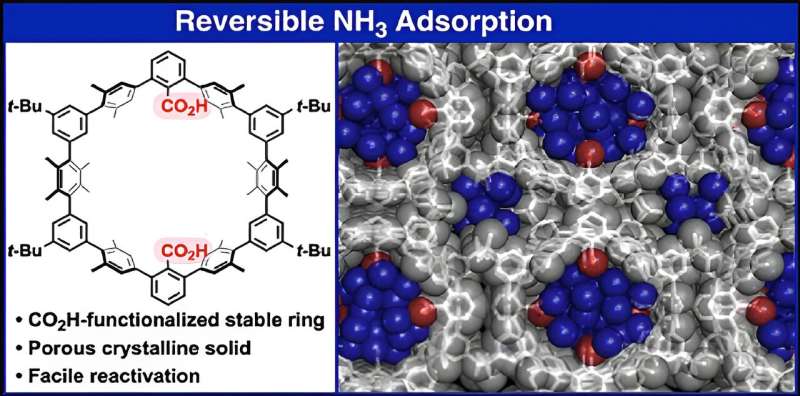
However, materials for NH3 storage should be chemically stable while also supporting energy-efficient ways of adsorbing and releasing captured gas. To realize such a material, the researchers created a crystalline solid out of 1a molecules, which are cyclic oligophenylenes with CO2H functional groups in the inner portion of their ring-like structure.
When forming this porous crystalline solid, referred to as 1a (N), the 1a molecules organize themselves into bundles of parallel nanochannels. Thanks to the CO2H groups, the channels are acidic, which in turn helps adsorb NH3. Worth noting, the packing density for NH3 in 1a (N) is 0.533 g/cm3 at room temperature—almost as high as the density of pure liquid NH3.
Interestingly, simply lowering the pressure around 1a (N) is enough to make it release almost all the stored NH3, which addresses a main limitation of previously reported materials.
"Crystalline 1a (N) is a stable NH3-adsorption material with the ability for repeated usage. The issue of residual NH3 during desorption, which has often plagued conventional NH3-adsorption materials, can be resolved when using 1a (N) via a simple decompression operation," says Ono. In addition to these qualities, 1a (N) is also easy to prepare, which extends its applicability and cost-effectiveness.
Overall, this innovation could serve as a much-needed stepping stone toward efficient and scalable NH3 storage, thereby paving the way to sustainable hydrogen economies. Moreover, by substituting the CO2H functional groups with different compounds, it may be possible to adsorb other types of highly reactive gases that typically pose practical challenges, such as HCl or Cl2.
More information: Kosuke Ono et al, Reversible Adsorption of Ammonia in the Crystalline Solid of a CO2H-Functionalized Cyclic Oligophenylene, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c03798
Journal information: Journal of the American Chemical Society
Provided by Tokyo Institute of Technology