This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Nature-inspired novel catalyst paves the way for efficient hydrocarbon decomposition
A research team affiliated with UNIST has developed a novel catalyst that mimics the ability of a natural enzyme to break down harmful hydrocarbons, paving the way for a more environmentally friendly and energy-efficient approach to reducing pollution.
Led by Professor Jaeheung Cho in the Department of Chemistry at UNIST, the research team has succeeded in creating a new catalyst that mimics the ability of metalloenzyme, which are ubiquitous in nature, to oxidize hydrocarbons.
The research has been published in the online version of Journal of the American Chemical Society on June 3, 2024.
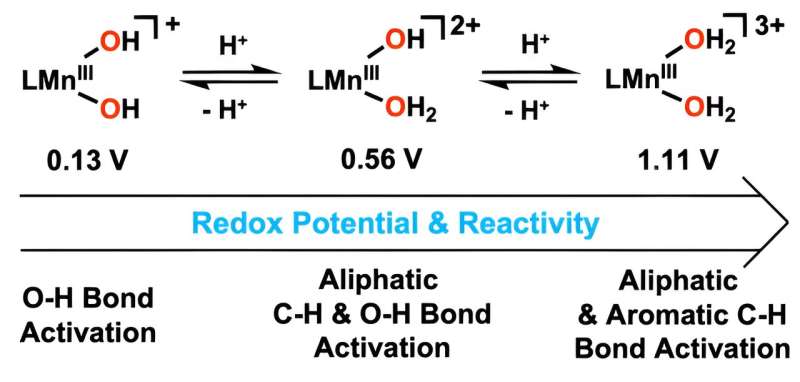
By adding hydrogen ions to the hydroxo ligand, metal-bound water molecules were synthesized. The new catalyst, which uses metal-bound water molecules, can oxidize carbon–hydrogen (C–H) bonds at lower temperatures than existing methods, making it a game-changer for the development of sustainable technologies.
The addition of hydrogen ions to the manganese catalyst has significantly improved its ability to activate oxygen–hydrogen bonds, resulting in accelerated reaction rates. This enhanced activity is attributed to the increased reduction potential of manganese, achieved by modifying the hydroxyl ligand to include water.
The new catalyst demonstrated its efficacy in oxidizing anthracene, a substance with strong carbon–hydrogen bonds, at low temperatures, effectively removing toxicity. Additionally, it was able to decompose aromatic hydrocarbons that are insoluble in water and chemically stable.
According to Professor Cho, "This is the first instance where a manganese(III) complex with two water molecules has reacted with aromatic hydrocarbons at low temperatures." He emphasized that controlling the reduction potential of manganese will contribute to the development of industrially important metal catalysts capable of breaking down strong carbon–hydrogen bonds.
More information: Yuri Lee et al, Controlling Redox Potential of a Manganese(III)–Bis(hydroxo) Complex through Protonation and the Hydrogen-Atom Transfer Reactivity, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c01927
Journal information: Journal of the American Chemical Society