This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
An outlandish molecule may be lurking inside Uranus and Neptune, affecting their magnetic fields
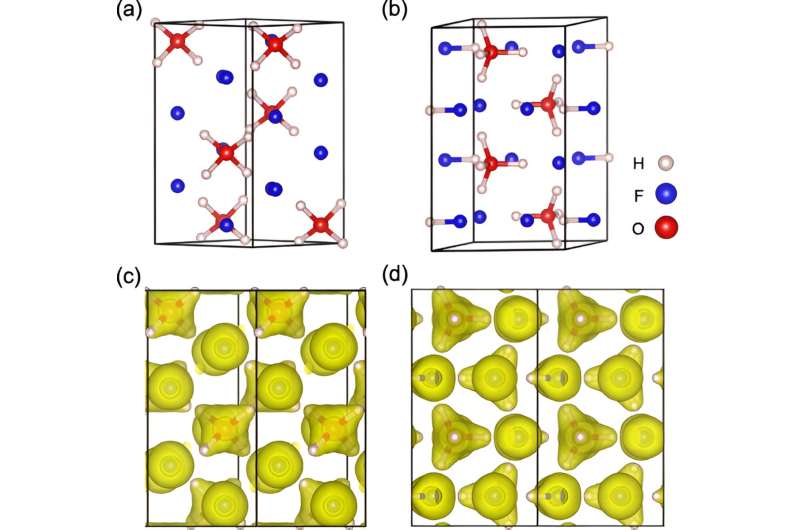
Skoltech scientists and their Chinese colleagues have determined the conditions that enable the existence of a very peculiar ion. Dubbed aquodiium, it can be conceptualized as an ordinary neutral molecule of water with two additional protons stuck to it, resulting in a net double positive charge.
The team suggests that the ion could be stable in the interior of the ice giants Uranus and Neptune, and if so, it must play a part in the mechanism that gives rise to these planets' unusual magnetic fields. The study is published in Physical Review B
Strange magnetism
The magnetic fields of Uranus and Neptune are not understood quite as well as those of Jupiter and Saturn—or our own planet, for that matter.
In the Earth's interior, circulation of the electronically conductive liquid iron-nickel alloy produces magnetism. Deep inside Jupiter and Saturn, hydrogen is thought to be pressed into a metallic state and give rise to magnetic fields in much the same way.
By contrast, the magnetic fields of Uranus and Neptune are hypothesized to stem from the circulation of ionically conductive media, where the constituent ions are themselves charge carriers, rather than merely a support structure enabling the flow of electrons.
If planetary scientists knew exactly what ions and in which proportions are involved, perhaps they could figure out why the ice giants' magnetospheres are so quirky: misaligned with the direction of the planets' rotation and offset from their physical centers.
Skoltech Professor Artem R. Oganov, who co-authored the paper, explains how ionic and electronic conductivity are different and where the newly predicted ion fits into this: "The hydrogen surrounding Jupiter's rocky core at those conditions is a liquid metal: It can flow, the way molten iron in the Earth's interior flows, and its electrical conductivity is due to the free electrons shared by all the hydrogen atoms pressed together.
"In Uranus, we think that hydrogen ions themselves—i.e., protons—are the free charge carriers. Not necessarily as standalone H+ ions, but perhaps in the form of hydronium H3O+, ammonium NH4+, and a series of other ions. Our study adds one more possibility, the H4O2+ ion, which is extremely interesting from the chemical viewpoint."
Missing link
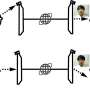
In chemistry, there's the notion of sp3 hybridization, which refers to the way electron orbitals combine with each other and amounts to something like a natural template for making plausible molecules and ions. Under sp3 hybridization, the nucleus of an atom—e.g., carbon, nitrogen, or oxygen—occupies the center point of an imaginary tetrahedron.
Each of the four vertices hosts either a valence electron or two paired electrons that are not available for making bonds with other atoms. The simplest example would be a carbon atom with four unpaired electrons at the four vertices—add four hydrogen atoms and you get a methane molecule: CH4.
For an oxygen atom, which has two electron pairs of its own in the outermost shell, along with two valence electrons, sp3 hybridization would mean only two of the vertices could host a covalent bond with hydrogen, with the remaining two occupied by electron pairs, which yields H2O, water.
If you attach a hydrogen ion (a proton) to one of the electron pairs, you get a hydronium ion H3O+, and this is actually what you get in an acid solution, because acids donate protons H+ into the solution and lone protons are immediately drawn to electron pairs.
Pressure and acid
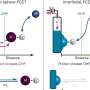
"But the question was: Can you add yet another proton to the hydronium ion to fill in the missing piece? Such a configuration at normal conditions is energetically very unfavorable, but our calculations show there are two things that can make it happen," says Professor Xiao Dong from China's Nankai University, whose original idea underlies this research.
"First, very high pressure compels matter to reduce its volume, and sharing a previously unused electron pair of oxygen with a hydrogen ion (proton) is a neat way of doing that: like a covalent bond with hydrogen, except both electrons in the pair come from oxygen. Second, you need lots of available protons, and that means an acidic environment, because that's what acids do—they donate protons."
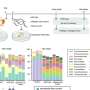
The team used advanced computational tools to predict what happens to hydrofluoric acid and water under extreme conditions. The result: Given a pressure of about 1.5 million atmospheres and a temperature around 3,000 degrees Celsius, well-separated aquodiium H4O2+ ions turn up in the simulation.
The scientists think that their newly discovered ion should play an important role in the behavior and properties of water-based media, specifically those under pressure and containing acid.
This roughly corresponds to conditions on Uranus and Neptune, where an immensely deep liquid water ocean produces extremely high pressures and some amount of acid might be expected, too. If so, aquodiium ions will form and by participating in the ocean's circulation, will contribute to these planets' magnetic fields and other properties in ways distinct from other ions.
Perhaps, aquodiium might even form as yet unknown minerals under those extreme conditions.
More information: ingyu Hou et al, H4O2+ ion stabilized by pressure, Physical Review B (2024). DOI: 10.1103/PhysRevB.109.174102
Journal information: Physical Review B
Provided by Skolkovo Institute of Science and Technology