This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study reveals a reaction at the heart of many renewable energy technologies
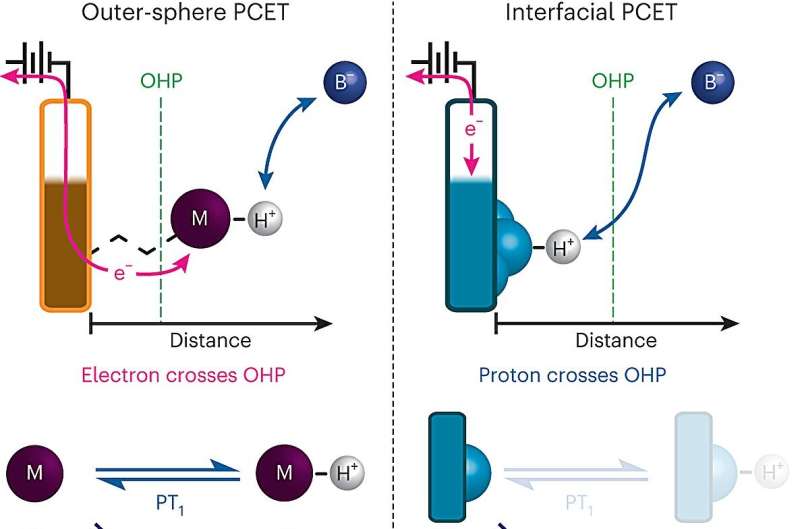
A key chemical reaction—in which the movement of protons between the surface of an electrode and an electrolyte drives an electric current—is a critical step in many energy technologies, including fuel cells and the electrolyzers used to produce hydrogen gas.
For the first time, MIT chemists have mapped out in detail how these proton-coupled electron transfers happen at an electrode surface. Their results could help researchers design more efficient fuel cells, batteries, or other energy technologies.
The research paper is published in the journal Nature Chemistry.
"Our advance in this paper was studying and understanding the nature of how these electrons and protons couple at a surface site, which is relevant for catalytic reactions that are important in the context of energy conversion devices or catalytic reactions," says Yogesh Surendranath, a professor of chemistry and chemical engineering at MIT and the senior author of the study.
Among their findings, the researchers were able to trace exactly how changes in the pH of the electrolyte solution surrounding an electrode affect the rate of proton motion and electron flow within the electrode.
MIT graduate student Noah Lewis is the lead author of the paper. Ryan Bisbey, a former MIT postdoc; Karl Westendorff, an MIT graduate student; and Alexander Soudackov, a research scientist at Yale University, are also authors of the paper.
Passing protons
Proton-coupled electron transfer occurs when a molecule, often water or an acid, transfers a proton to another molecule or to an electrode surface, which stimulates the proton acceptor to also take up an electron. This kind of reaction has been harnessed for many energy applications.
"These proton-coupled electron transfer reactions are ubiquitous. They are often key steps in catalytic mechanisms, and are particularly important for energy conversion processes such as hydrogen generation or fuel cell catalysis," Surendranath says.
In a hydrogen-generating electrolyzer, this approach is used to remove protons from water and add electrons to the protons to form hydrogen gas. In a fuel cell, electricity is generated when protons and electrons are removed from hydrogen gas and added to oxygen to form water.
Proton-coupled electron transfer is common in many other types of chemical reactions; for example, carbon dioxide reduction (the conversion of carbon dioxide into chemical fuels by adding electrons and protons). Scientists have learned a great deal about how these reactions occur when the proton acceptors are molecules, because they can precisely control the structure of each molecule and observe how electrons and protons pass between them.
However, when proton-coupled electron transfer occurs at the surface of an electrode, the process is much more difficult to study because electrode surfaces are usually very heterogenous, with many different sites to which a proton could potentially bind.
To overcome that obstacle, the MIT team developed a way to design electrode surfaces that gives them much more precise control over the composition of the electrode surface. Their electrodes consist of sheets of graphene with organic, ring-containing compounds attached to the surface. At the end of each of these organic molecules is a negatively charged oxygen ion that can accept protons from the surrounding solution, which causes an electron to flow from the circuit into the graphitic surface.
"We can create an electrode that doesn't consist of a wide diversity of sites but is a uniform array of a single type of very well-defined sites that can each bind a proton with the same affinity," Surendranath says. "Since we have these very well-defined sites, what this allowed us to do was really unravel the kinetics of these processes."
Using this system, the researchers were able to measure the flow of electrical current to the electrodes, which allowed them to calculate the rate of proton transfer to the oxygen ion at the surface at equilibrium—the state when the rates of proton donation to the surface and proton transfer back to solution from the surface are equal. They found that the pH of the surrounding solution has a significant effect on this rate: The highest rates occurred at the extreme ends of the pH scale—pH 0, the most acidic, and pH 14, the most basic.
To explain these results, researchers developed a model based on two possible reactions that can occur at the electrode. In the first, hydronium ions (H3O+), which are in high concentration in strongly acidic solutions, deliver protons to the surface oxygen ions, generating water. In the second, water delivers protons to the surface oxygen ions, generating hydroxide ions (OH-), which are in high concentration in strongly basic solutions.
However, the rate at pH 0 is about four times faster than the rate at pH 14, in part because hydronium gives up protons at a faster rate than water.
A reaction to reconsider
The researchers also discovered, to their surprise, that the two reactions have equal rates not at neutral pH 7, where hydronium and hydroxide concentrations are equal, but at pH 10, where the concentration of hydroxide ions is 1 million times that of hydronium. The model suggests this is because the forward reaction involving proton donation from hydronium or water contributes more to the overall rate than the backward reaction involving proton removal by water or hydroxide.
Existing models of how these reactions occur at electrode surfaces assume that the forward and backward reactions contribute equally to the overall rate, so the new findings suggest that those models may need to be reconsidered, the researchers say.
"That's the default assumption, that the forward and reverse reactions contribute equally to the reaction rate," Surendranath says. "Our finding is really eye-opening because it means that the assumption that people are using to analyze everything from fuel cell catalysis to hydrogen evolution may be something we need to revisit."
The researchers are now using their experimental setup to study how adding different types of ions to the electrolyte solution surrounding the electrode may speed up or slow down the rate of proton-coupled electron flow.
"With our system, we know that our sites are constant and not affecting each other, so we can read out what the change in the solution is doing to the reaction at the surface," Lewis says.
More information: Noah B. Lewis et al, A molecular-level mechanistic framework for interfacial proton-coupled electron transfer kinetics, Nature Chemistry (2024). DOI: 10.1038/s41557-023-01400-0
Journal information: Nature Chemistry
Provided by Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.