How confined protons migrate

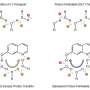
Protons (H+) and hydronium ions (H3O+) in free aqueous solutions seem to migrate faster than other ions due to the the Grotthuss-mechanism. Individual protons do not really migrate at all. Instead, bonds of the hydronium ions are broken and new bonds to other water molecules are formed, so that the individual proton does not migrate. Rather charges are transported directly from one water molecule to the next. This process is quicker than the diffusion of an ion through the solution.
Behavior in confined spaces unexplored
So far, many studies have investigated the transport of protons in free aqueous solution. "In real life such conditions are relatively rare," says Professor Martina Havenith, speaker of RESOLV and an author of the study. "Most protons transport processes actually occur in confined spaces or in nanopores." Hydronium ions are involved in defining the pH value. Up to now, the effect the of confinement has not yet been completely understood.
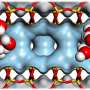
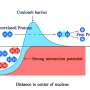
To change that, researchers from Bochum and Berkeley combined theoretical and experimental methods. They created tiny water pools, whose size could be precisely controlled. As soon as the diameter of the droplets became smaller than two nanometers, the proton transport mechanism in the experiment and simulations changed abruptly. "Under two nanometers the proton migration is restricted by confinement effects. This effect is reduced when the water pool is enlarged," explains Martina Havenith. "Suprisingly we found that above two nanometres, where the formation of hydronium ions is possible, there is a proton traffic jam." The proton is stuck in an oscillatory state, where it bounces back and forth along the surface of the water pool, but makes no progress forward, resulting in the conductivity not increasing further—as originally expected.
Short-circuit in the hydrogen-bonding network
In addition to the size of the pools, the acid concentration also influences the proton migration behavior. When the research team increased the acid content, they created a type of short-circuit in the hydrogen bonding network of the droplet, so that the proton no longer migrated from its position, but rather paused in oscillatory bouncing state. "That has consequences for every system that relies on proton transport, because the size of the system or the proton concentration can lead to a traffic jam and for example disrupt the signaling process," concludes Havenith.
More information: Martina Havenith-Newen et al, Proton Traffic Jam: Effect of Nanoconfinement and Acid Concentration on Proton Hopping Mechanism, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202108766
Journal information: Angewandte Chemie International Edition
Provided by Ruhr-Universitaet-Bochum