This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New method for light-driven oxidation of aryl ethers to esters
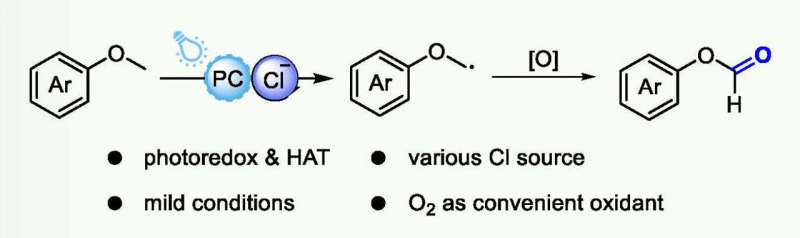
Oxidation of saturated C−H bonds is a key chemical reaction in synthetic chemistry and the chemical industry. Nevertheless, the high bond dissociation energies (BDEs) and weak polarity of C(sp3)−H bonds, especially in saturated hydrocarbons, lead to weak interactions with many catalysts and difficult substrate activation. Aryl ethers are ubiquitous structural motifs in natural products and pharmaceuticals, including dozens of small molecule drugs in the top 200 retail sales.
Although the C(sp3)−H bond of aryl ethers can be used as a synthon for organic synthesis to construct C−C/C−N bonds, its oxidative functionalization and application are still limited and challenging. Therefore, it is necessary to develop efficient and convenient methods for the functionalization of ether C(sp3)−H bonds and their utilization in organic synthesis and pharmaceutical chemistry.
At present, photocatalytic C(sp3)−H oxidation has developed into a useful and diverse tool for catalysis research due to its convenient and redox-neutral manner. Hydrogen atom transfer (HAT) is an effective strategy to cleave C(sp3)–H bonds of alkane feedstocks. And chlorine radicals are used as a powerful HAT reagent in a wide variety of oxidation reactions due to their high oxidative capacity (E1/2red = +2.03 V vs. SCE), which can abstract hydrogen of aryl ether C(sp3)−H bond to form the corresponding alkyl radical.
Nevertheless, chlorine radicals are not readily available due to unfavorable chloride-to-chlorine oxidation (Eo=1.36 V vs. NHE). Methods such as the photolysis of Cl2 or photoinduced ligand-to-metal charge transfer (LMCT) have been reported for chloride-to-chlorine generation, but the strategy development for more extensive chlorine sources generating chlorine radicals and promoting C(sp3)−H bond oxidation remains attractive.
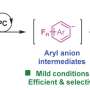
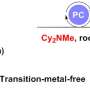
Recently, a research team led by Prof. Feng Wang from Dalian Institute of Chemical Physics, Chinese Academy of Sciences, reported a novel method of visible-light-driven C(sp3)−H bond oxidation of aryl ethers selectively into ester products using oxygen as the oxidant. During the photocatalytic reaction using Mes-10-phenyl-Acr+−BF4- catalyst, chlorine radicals are generated from a wide variety of chloride sources and can effectively activate aryl ether C(sp3)−H bonds into alkyl radicals through the hydrogen atom transfer (HAT) process.
Aryl ethers with different substituents can be oxidized to esters in good to excellent yields. This work presents a new photocatalytic strategy for C(sp3)−H oxidation of aryl ethers in a convenient and green manner.
The research is published in the Chinese Journal of Catalysis.
More information: Yuting Liu et al, Chlorine radical-mediated photocatalytic C(sp3)−H bond oxidation of aryl ethers to esters, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64615-7
Provided by Chinese Academy of Sciences