Mechanism of Cl-initiated oxidation of methacrolein under NOx-free conditions
Chlorine atoms (Cl) are more reactive in the atmosphere than other oxidants. In recent years, researchers have seen increased concentrations of CI precursors in inland areas. The atmospheric oxidation reaction caused by Cl is becoming more and more important.
Methylacrolein (MACR) is a key intermediate in atmospheric oxidation of biogenic isoprene. The oxidation and degradation of MACR play an essential role in the formation of atmospheric ozone and secondary organic aerosols.
A team of researchers led by Prof. Zhang Weijun from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences (CAS), recently investigated Cl-initiated oxidation reactions of MACR under nitrogen oxides (NOx)-free conditions, by using a home-made photoionization time-of-flight mass spectrometer complemented with theoretical calculations.
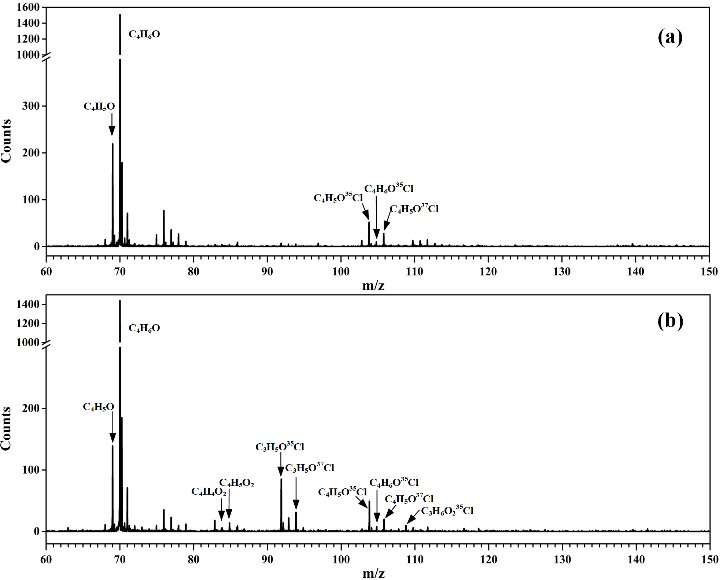
In this study, the researchers used a microwave discharge flow tube reactor to investigate the oxidation reaction of Cl + MACR. Key species such as intermediate radicals and products during the oxidation process were detected online and confirmed in photoionization mass spectra.
The results showed that the reaction of MACR with Cl atoms could generate the C4H5O and C4H6OCl radicals via hydrogen abstraction and the addition of Cl atom to the C=C double bond, respectively.
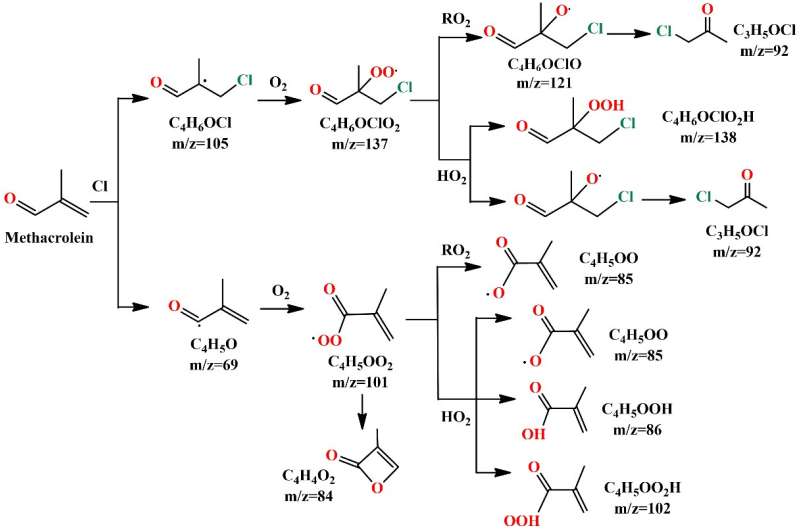
"This is the first time that the transient C4H5O and C4H6OCl radicals are experimentally detected here," said Lin Xiaoxiao, first author of the study.
The C4H5O and C4H6OCl radicals could react with oxygen to produce the corresponding peroxy radicals C4H5OO2 and C4H6OClO2. Under low NOx conditions, these peroxy radicals would perform bimolecular reactions with themselves and the HO2 radicals.
Combined with theoretical calculation, the specific products obtained can be identified in the photoionization mass spectrometry.
This work elucidates the chemical mechanisms of Cl-initiated oxidation of MACR, which is helpful to understand the chemical behavior of MACR in the atmosphere.
More information: Xiaoxiao Lin et al, Cl-Initiated oxidation of methacrolein under NOx-free conditions studied by VUV photoionization mass spectrometry, Physical Chemistry Chemical Physics (2022). DOI: 10.1039/D2CP02101C
Journal information: Physical Chemistry Chemical Physics
Provided by Chinese Academy of Sciences