This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unlocking complex sulfur molecules: A novel approach for synthesis of functionalized benzenethiol equivalents
Organosulfur compounds, organic compounds containing sulfur, are vital in biological processes and research fields like pharmaceuticals, biomedical imaging, agriculture, and electronics. Compounds like phenothiazine, thianthrene and thienothiophene, containing organosulfur skeletons, play pivotal roles in these domains.
Multiple methods have been developed that use o-bromobenzenethiols as the key component to synthesize these compounds. This is mainly owing to the high reactivities of the thiol (sulfur atom bonded to a hydrogen atom) and bromine moieties of o-bromobenzenethiols, compounds which contain a bromine atom attached to positions one or two (ortho position) in a benzene ring next to a sulfur atom.
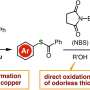
Conventional methods to produce o-bromobenzenethiols involve addition of bromine to aniline, then using diazonium intermediates for addition of sulfur. This process poses challenges including selective addition of bromine at the ortho position, difficulty working with certain chemical groups, and the tendency of o-bromobenzenethiols to oxidize easily in air releasing unpleasant odors.
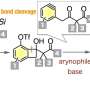
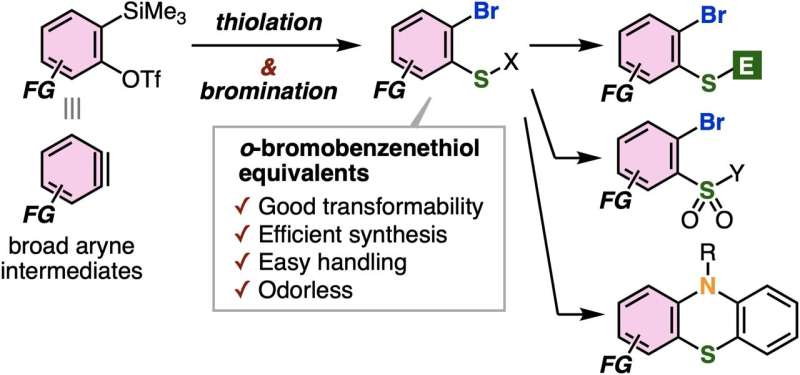
Recently, scientists have found a new method with great potential to synthesize various complex aromatic compounds by selectively adding molecules on aryne intermediates. However, despite this success, creating o-bromobenzenethiols by adding a bromine molecule and a thiol group to these intermediates has been tough. This is because the sulfur-containing compounds react strongly with the aryne molecules, making it hard to control the process.
To tackle this challenge, Associate Professor Suguru Yoshida and Mr. Shinya Tabata, from the Department of Biological Science and Technology, Faculty of Advanced Engineering at Tokyo University of Science, have developed a new method.
Prof. Yoshida explains, "We developed a method for synthesizing stable o-bromobenzenethiol equivalents by bromothiolation of aryne intermediates with an appropriate hydrogen sulfide equivalent and an electrophilic brominating reagent, resulting in controlled reactivity at the sulfur atom which prevents subsequent additions with aryne intermediates."
The findings are published in the journal Organic Letters.
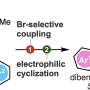
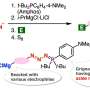
The researchers utilized potassium xanthate instead of hydrogen sulfide, owing to its better reactivity in aryne reactions and pentafluorophenyl bromide as an electrophilic brominating reagent. This combination allowed them to efficiently produce complex o-bromobenzenethiol equivalents called aryl xanthates.
Notably, this method demonstrated good tolerance for various chemical groups and prevented the formation of unwanted products. Using aryl xanthates, the researchers prepared diverse highly functionalized organosulfur compounds, such as phenothiazines and thianthrenes, utilizing simple protocols without any foul odor, thanks to the high stability of the compounds.
With readily available aryne precursors and versatile transformations achievable using o-bromobenzenethiols, this method enabled the synthesis of a wide variety of organosulfur compounds with highly fused organosulfur skeletons. Furthermore, this method holds the potential to significantly reduce the number of steps in the synthesis of multi substituted organosulfurs contributing towards drug discovery in pharmaceutical sciences.
"With our method, it is now possible to synthesize sulfur-containing compounds with complex structures that are difficult to achieve using conventional methods. It can enable the development of novel organosulfur compounds that could lead to the discovery of new drugs, eco-friendly agrochemicals for sustainable agriculture, advanced materials, and organic electronics," remarks Prof. Yoshida, underscoring the potential applications of their study.
This study opens new possibilities for the synthesis of organosulfur compounds with diverse applications in many fields. "Further studies on expansion of scope and applications of this method for synthesis of bioactive organosulfur compounds are underway in our laboratory," concludes Prof. Yoshida.
More information: Shinya Tabata et al, Bromothiolation of Arynes for the Synthesis of 2-Bromobenzenethiol Equivalents, Organic Letters (2024). DOI: 10.1021/acs.orglett.4c00944
Journal information: Organic Letters
Provided by Tokyo University of Science