This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Extending the playing field for organosulfur: A new way to synthesize sulfonate esters
Organosulfur compounds are organic molecules that contain one or more sulfur atoms bonded to carbon atoms. They not only play fundamental roles in biological processes but also have wide applications in many industries, such as pharmaceuticals, agrochemicals, and materials science. Thus, many chemists strive to develop safe and efficient methods to synthesize organosulfurs.
The conventional approach to produce them involves the oxidation of molecules called thiols. However, working with thiols can be quite challenging. They have a strong and unpleasant odor and can be oxidized easily under air, which makes handling and storage difficult. These two issues have limited the availability of thiols with interesting functional groups, also hindering the production of different types of organosulfur. But what if we could produce organosulfurs from less problematic chemicals?
In a recent study published in Organic and Biomolecular Chemistry, a research team from Japan has come up with a new approach to synthesize sulfonate esters, a subclass of organosulfur compounds, using thioesters. The research, led by Associate Professor Suguru Yoshida, is co-authored by Mr. Keisuke Nakamura, Ms. Yukiko Kumagai, Mr. Akihiro Kobayashi, and Ms. Minori Suzuki, all from Tokyo University of Science (TUS).
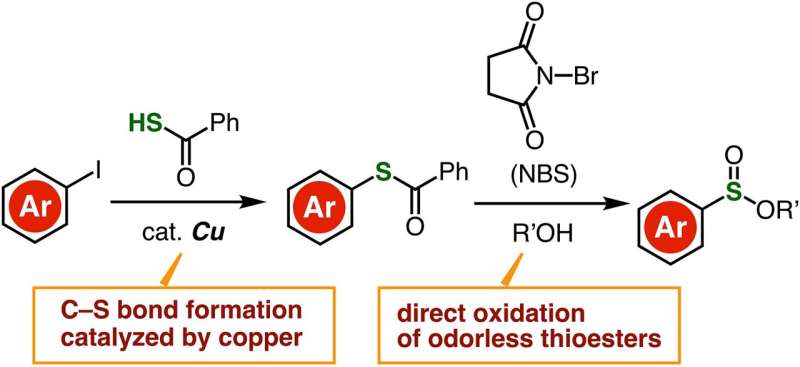
Thioesters have essentially the same chemical structure as esters, except that one or two oxygen atoms are replaced by sulfur atoms. Unlike thiols, thioesters are odorless, stable, and easily accessible, which makes them easier to work with. These advantages motivated the research team to develop an efficient synthesis route for the synthesis of sulfonate esters via direct oxidation of thioesters.
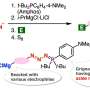
They first prepared a desired thioester molecule from an aryl iodide composed of an aryl group bound to an iodine atom. Using a copper-containing catalyst, the researchers managed to strip the iodine atom from the aryl group and replace it with a carbon–sulfur bond, forming a thioester. Afterwards, the thioester was directly oxidized in the presence of N-bromosuccinimide, which created an intricate reaction pathway culminating with the formation of a sulfonate ester.
This two-step synthesis technique is efficient and straightforward. Most importantly, it carries the potential to produce various sulfinate esters from easily available starting materials, including carboxylic acids, anilines, and a wide variety of aryl iodides. "Compared to conventional preparation methods of sulfinate esters from other sulfur surrogates, the superior accessibility of aryl iodides from a wide variety of aromatic compounds will enable the synthesis of highly functionalized sulfinate esters," remarks Dr. Yoshida.
Overall, the method proposed in this study will greatly bolster research on new organosulfurs, leading to promising applications in many fields. For example, sulfinate esters are used in the synthesis of sulfonamide-containing compounds, which have antimicrobial, anti-inflammatory, and enzyme inhibitory activities.
They are also used to produce drugs with sulfoxide groups, which can have various biological activities, including anti-clotting and anti-acid effects. Moreover, sulfinate esters can help synthesize functional polymers and agrochemicals and serve as reagents in analytical chemistry techniques to detect the presence of specific compounds or functional groups.
Dr. Yoshida concludes, "Further studies towards finding applications for the preparation of bioactive organosulfur derivatives, as well as the synthesis of bis-sulfinate esters, are underway in our laboratory."
More information: Keisuke Nakamura et al, Facile synthesis of sulfinate esters from aryl iodides via direct oxidation of thioesters, Organic & Biomolecular Chemistry (2023). DOI: 10.1039/D3OB01108A
Provided by Tokyo University of Science