This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Green and efficient approach to synthesizing esters for flavorings, fragrances
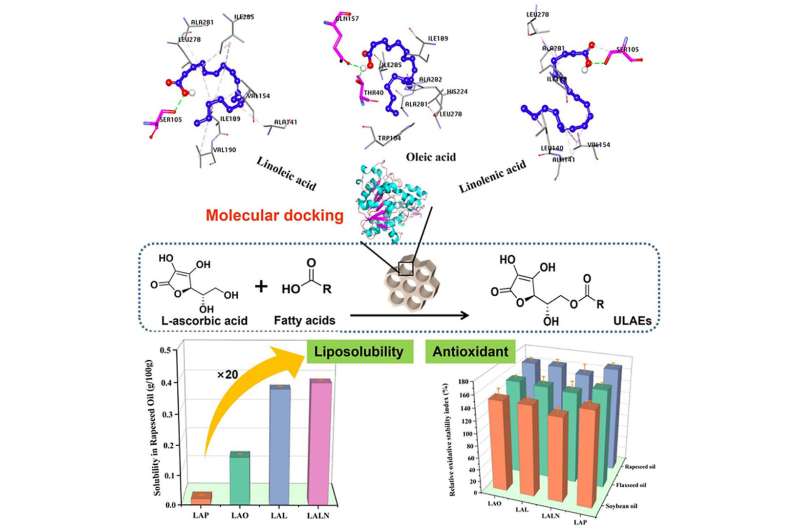
Scientists from Oil Crops Research Institute (OCRI) of Chinese Academy of Agricultural Sciences (CAAS), Anhui Agricultural University (Anhui, China), Newcastle University in Singapore, and Huizhou Comvikin Biotechnology Co., Ltd (Guangdong, China) have developed a green and efficient approach to synthesize highly liposoluble and antioxidant L-ascorbyl esters by immobilized lipases.
An ester is a type of chemical compound that is formed by the reaction between an alcohol and a carboxylic acid. It has a characteristic sweet and fruity odor and is used in the manufacture of flavors, fragrances, and plastics. Esters are also found in many natural substances such as fats, oils, and waxes.
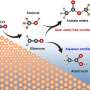
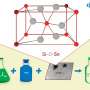
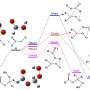
The permitted antioxidant in baby food is L-ascorbyl palmitate, but its low liposolubility limits its commercial demand. A team of researchers, led by Professor Mingming Zheng and Dr. Yi Zhang from Oil Crops Research Institute, has proposed a more efficient and environmentally-friendly method for synthesizing highly liposoluble unsaturated fatty acid ascorbyl esters using enzymatic catalysis by Lipase CaLB immobilized on ordered mesoporous silicon.
In this approach, self-made immobilized lipases are used to catalyze the reaction that produces L-ascorbyl esters. These lipases are immobilized, meaning they are attached throughout the mesoporous carrier's surface, which improves the catalytic stability and reusability of the lipases. Using unsaturated fatty acids as acyl donors results in the synthesized ascorbyl esters having better liposolubility (i.e., they can dissolve in fats and oils), a lower melting point, and comparable antioxidant activity to L-ascorbyl palmitate.
Compared to traditional synthesis methods, this novel approach that utilizes enzymatic esterification is a more effective and cleaner method of synthesizing highly-soluble and antioxidant L-ascorbyl esters. It holds significant potential for implementation in industrial production.
The team leaders have submitted an application to patent this novel approach.
The work has been published in the Journal of Cleaner Production.
More information: Tiantian Zhang et al, Green and efficient synthesis of highly liposoluble and antioxidant L-ascorbyl esters by immobilized lipases, Journal of Cleaner Production (2022). DOI: 10.1016/j.jclepro.2022.134772
Journal information: Journal of Cleaner Production
Provided by Newcastle University in Singapore