A reusable catalyst for the synthesis of esters
A chemist from RUDN University has developed a tin silicate catalyst for the production of esters—flavourings, plasticisers, and biofuel components. Unlike existing catalysts, the new material can be made active again and reused. The results are published in the journal Microporous and Mesoporous Materials.
Catalysts are not consumed in the process of chemical reactions, yet it is difficult in some cases to separate them from the synthesis waste and reuse them. For instance, inorganic acid catalysts are used for esterification, i.e. to obtain esters from organic acids and alcohol. In this case, the final product of reaction must be purified and the waste disposed of, along with the catalysts, since it is more expensive to separate them for reuse than to acquire new ones.
One promising solution is solid catalysts based on tin ions deposited on a porous support substrate. Its "active centres" are located on its surface: ions on which a chemical transformation occurs, for example, the formation of ether. However, tin ions are "washed out" during the use of such materials, and they lose their activity. Moreover, a lot of useless tin oxide is formed during the manufacture of the catalyst, in addition to ions.
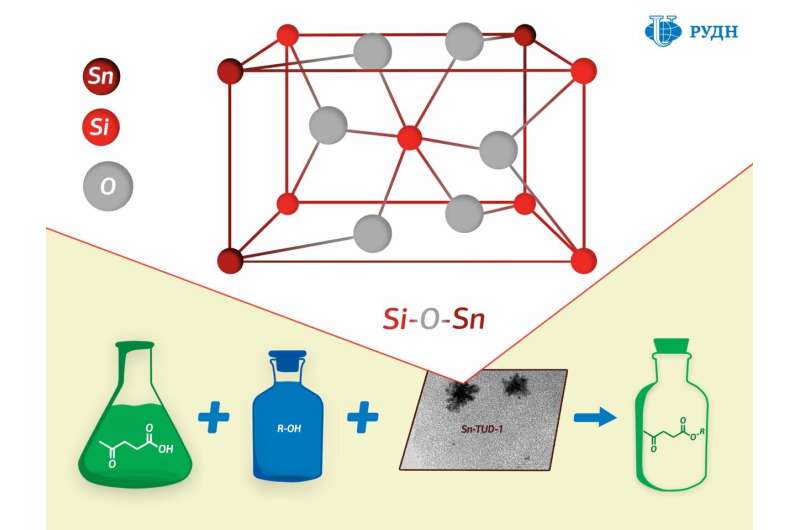
RUDN University chemist Rafael Luque has developed a new catalyst production method that results in a porous silicate matrix with "embedded" tin ions (Sn4+) held together by strong chemical bonds.
"It allows the design of highly active and selective Sn-based materials for acid catalysed processes—not only for levulinic acid esterification—that can be also reused, being highly stable under moderate temperatures and pressures," said Luque.
While the existing methods for creating such catalysts involve tin being applied to a finished porous matrix of silicon dioxide, professor Luque formed the catalyst "from scratch." The silicon dioxide substrate in his experiment was formed from a precursor (tetraethoxysilane) in the presence of tin, due to which tin ions were embedded in the chemical structure of the substrate.
The study of the substrate using XPS (X-ray photoelectron spectroscopy) showed that a chemical bond of silicon oxide and tin (Si–O–Sn) indeed formed in the catalyst.
The surface area of 1 gram of catalyst is significant—it's 600 square metres. Since chemical reactions occur on the surface of a catalyst, the larger its surface area, the higher the activity. Most catalysts based on a silicon matrix have a useful area two to three times smaller: about 200-300 square metres per gram.
Chemists tested the activity of the new catalyst in the synthesis of levulinic acid esters. Levulinic acid is a product of the processing of carbohydrates such as glucose and starch. When interacting with alcohols it forms esters, which can be used as flavorings, plasticisers, and components of biofuels. It turned out that the new catalyst allows obtaining esters of levulinic acid with a maximum product yield of 44 to 99 percent—the figure corresponds to the efficiency of most commonly used catalysts.
In addition, the catalyst was tested for reusability—the experiment showed that its activity did not decrease after five regenerations.
"In principle, the use of the catalyst can be extended to other acid catalysed reactions including isomerisations, etherifications, etc. for the production of compounds of interest for the fine chemicals industries (flavourings, odorants, pharmaceuticals) and even in the petrochemical industry. The advantages of the proposed approach include simplicity, relatively cheap nature of catalyst, reusability and stability and versatility as compared to others previously implemented," Luque noted.
More information: M.P. Pachamuthu et al. Preparation of mesoporous stannosilicates SnTUD-1 and catalytic activity in levulinic acid esterification, Microporous and Mesoporous Materials (2019). DOI: 10.1016/j.micromeso.2019.05.061
Provided by RUDN University