This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers investigate the surface extraction of platinum catalysts in alkaline media
Platinum (Pt) electrodes are crucial for clean power technologies like hydrogen fuel cells and electrolysis. However, the surface oxidation that occurs during such processes degrades catalyst performance and stability.
To address this, researchers investigated the mechanisms of surface oxidation on Pt surface in alkaline media, a previously unexplored avenue of research. Their experiments revealed crucial insights that can aid in the development of next-generation catalysts, paving the way for a carbon-neutral society. The findings are published in the Journal of the American Chemical Society.
The pursuit of carbon neutrality drives the exploration of clean energy sources, with hydrogen fuel cells emerging as a promising avenue. In these cells, hydrogen undergoes an electrochemical reaction with oxygen to produce electricity and water. Also, the reverse of this process, called electrolysis, can be used to split the abundantly available water to produce hydrogen and oxygen.
These two technologies can work in tandem to provide a clean and renewable source of energy. A pivotal element in these two technologies is the platinum (Pt) electrode.
Hydrogen fuel cells consist of two electrodes: an anode and a cathode, with an electrolyte between them. Pt serves as a fundamental catalyst in low-temperature fuel cells, such as alkaline fuel cells and polymer electrolyte fuel cells (PEFCs). Pt has a high activity for the oxygen reduction reaction (ORR), which is crucial for fuel cells, in alkaline and acidic conditions at the operating voltage of PEFC cathodes.
However, this also leads to oxide formation on the surface, which roughens and dissolves the Pt layer, ultimately degrading the cathodes and affecting performance and stability. Understanding surface oxide formation mechanisms is thus crucial for developing Pt cathode catalysts that work well in alkaline conditions.
Studies have shown that the oxide formation on the Pt surface depends on the electrode potential, the electrolyte, and the electrical double layer (EDL). While studies have investigated the oxide formation and reduction on the Pt surface in acidic media, few of them have addressed the same in alkaline media, present in fuel cells and electrolyzers with anion exchange membranes.
To address this gap, a team of researchers led by Professor Masashi Nakamura from the Graduate School of Engineering, Chiba University, Japan, dug deep into the oxide formation mechanisms on Pt surfaces in alkaline media.
"In a previous study, we reported that interfacial hydrophobic ions with long alkyl chains can enhance ORR. This suggests that it is possible to construct an interfacial reaction field that not only activates the ORR but also improves the durability of Pt electrodes by using optimal interfacial ions," explains Prof. Nakamura.
The study also included contributions from Dr. Tomoaki Kumeda and Professor Nagahiro Hoshi, both from the Graduate School of Engineering at Chiba University, along with Dr. Osami Sakata from the Center for Synchrotron Radiation Research at Japan Synchrotron Radiation Research Institute.
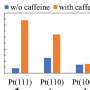
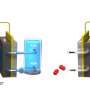
The team investigated the oxide formation on the Pt (111) surface in alkaline aqueous solutions containing different cations, namely Lithium cation (Li+), Potassium (K+) cation and Tetramethylammonium cation (TMA+), using advanced methods like X-ray crystal truncation rod (CTR) scattering, gold nanoparticle-based surface-enhanced Raman spectroscopy (GNP-SERS), and infrared reflection absorption spectroscopy (IRAS).
"Studies have shown that a combination of vibrational spectroscopy and X-ray diffraction is effective for elucidating surface oxidation processes," adds Prof. Nakamura.
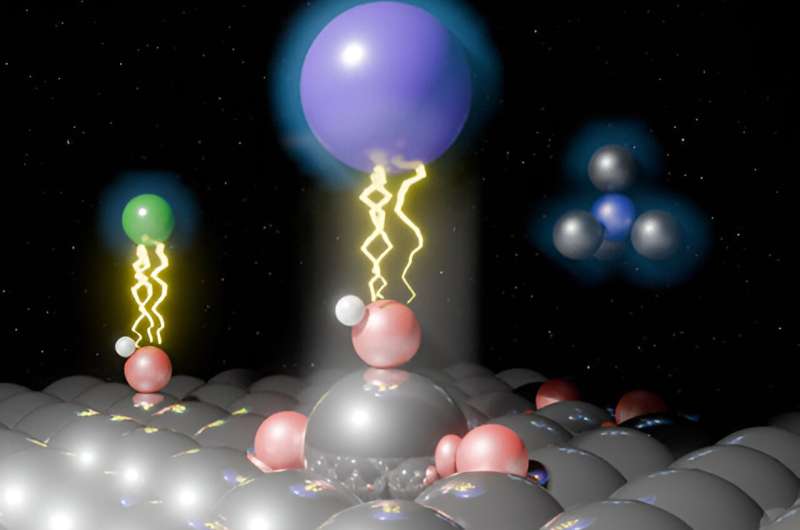
X-ray CTR revealed that oxide formation results in surface buckling and Pt extraction. SERS and IRAS measurements revealed the potential and cation-dependent formation of three oxide species, namely infrared (IR)-active adsorbed hydroxide OH (OHad), Raman active adsorbed water (H2O)ad, and Raman-active oxygen (Oad).
The team found that hydrophilic cations like Li+ stabilize IR-active OHad, thus preventing harmful oxide formation, while moderate hydrophilicity of K+ has no protective effect. Interestingly, bulky hydrophobic cations such as TMA+ also reduce irreversible oxidation, similar to Li+. Notably, the team also found that the electrostatic repulsion between Raman-active (H2O)ad and neighboring Raman-active Oad facilitates Pt extraction.
These results suggest that interfacial cations play an essential role in oxide formation on Pt surfaces, which can be controlled by selecting appropriate cations. Elaborating on these results, Prof. Nakamura remarks, "These insights are crucial for understanding the surface oxidation mechanisms and the EDL structure, which can be beneficial for achieving high-performance and stable Pt electrocatalysts for use in next-generation electrochemical devices."
Overall, this study takes us a step further in achieving a zero-carbon future powered by abundant and clean hydrogen.
More information: Tomoaki Kumeda et al, Surface Extraction Process During Initial Oxidation of Pt(111): Effect of Hydrophilic/Hydrophobic Cations in Alkaline Media, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c11334
Journal information: Journal of the American Chemical Society
Provided by Chiba University