This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Using an AuNi alloy on Au electrodes for a better hydrogen evolution reaction
In recent years, hydrogen gas has gained momentum as the fuel for a clean and green future. This carbon-neutral fuel source releases huge amounts of energy via combustion in the presence of oxygen with water vapor as the by-product. One of the most popular methods of hydrogen production is the splitting of water into hydrogen and oxygen using electricity.
An electrochemical cell is used to split water, and the hydrogen gas gets released at the negatively charged electrode in hydrogen evolution reaction (HER). Catalysts are used to lower the HER overpotential—the difference between the theoretical cell voltage and the voltage required to carry out hydrogen evolution—for making the process more efficient.
Recently, an alloy of gold (Au) and nickel (Ni) showed promising HER activity. While the electrochemical properties of AuNi have been well studied, not much is known about its surface structure and atomic composition, which dictate the electrocatalytic activity of a catalyst.
Now, a team of researchers from Chiba University, led by Associate Professor Masashi Nakamura from the Graduate School of Engineering and including doctoral student Syunnosuke Tanaka from the Graduate School of Science and Engineering and Professor Nagahiro Hoshi from the Graduate School of Engineering, has bridged the gap in the understanding of AuNi electrocatalysts.
In their recent breakthrough article published in ChemElectroChem, the team investigated the surface structure, atomic arrangement, and HER activity of AuNi surface alloys prepared at different alloying temperatures on single-crystal Au electrodes.
Dr. Nakamura discusses the motivation behind the present research. "Rare and highly expensive metals like platinum are commonly used as catalysts for water electrolysis. While Au shows high chemical stability as a catalyst compared to platinum, it suffers from low HER activity. Now, AuNi nanoparticles have emerged as a promising non-platinum alternative, and it is crucial to improve their HER activity further."
The team transferred the AuNi/Au electrode to an electrochemical cell with 0.05 M sulfuric acid to perform cyclic voltammogram (CV) and linear sweep voltammogram (LSV) measurements, evaluating its HER activity. The surface properties of the AuNi/Au catalyst were also analyzed using X-ray photoelectron spectroscopy (XPS) and surface X-ray diffraction (SXRD) techniques.
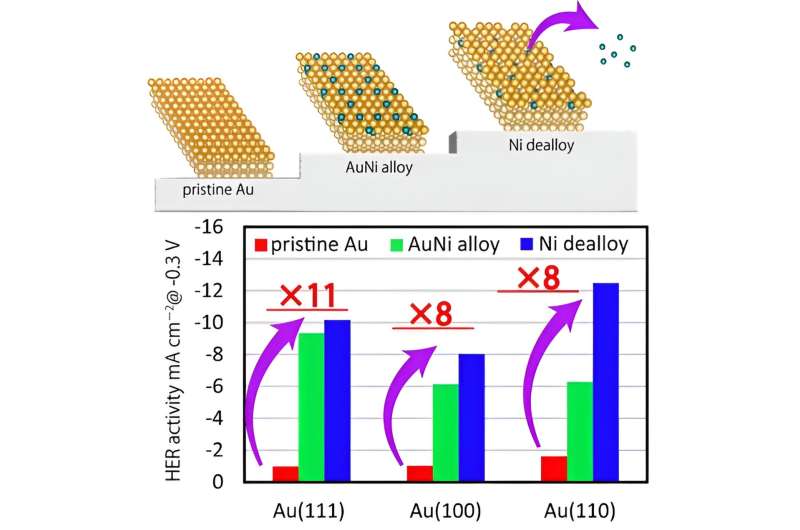
CVs and LSVs revealed that the HER activity of AuNi/Au depended on the surface structure of the Au substrate, with (110) surface resulting in the highest activity followed by (111) and (100), respectively.
Also, the surface alloy improved the HER activity via Ni dealloying. This was verified by XPS and SXRD, wherein the team observed a decrease in the atomic occupancy on the topmost layer of the surface caused by the dissolution of Ni from the surface-alloy layer. The Ni dealloying process created defects on the surface, and the low-coordination Au sites adjacent to Ni activated the HER.
The present study provides insights into the structural and electrochemical properties of AuNi surface alloy, paving the way for highly active and durable Au-based catalysts for practical electrolysis and fuel cell applications. "Designing effective non-platinum electrocatalysts can reduce the cost of water electrolysis and also improve its energy conversion efficiency, which is crucial for accelerating toward a hydrogen-driven society," concludes Dr. Nakamura.
More information: Syunnosuke Tanaka et al, Hydrogen Evolution Reaction on AuNi Surface Alloy Formed on Single Crystal Au Electrodes, ChemElectroChem (2023). DOI: 10.1002/celc.202300095
Provided by Chiba University