This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers reveal interfacial confinement on open space of oxide–oxide catalysts
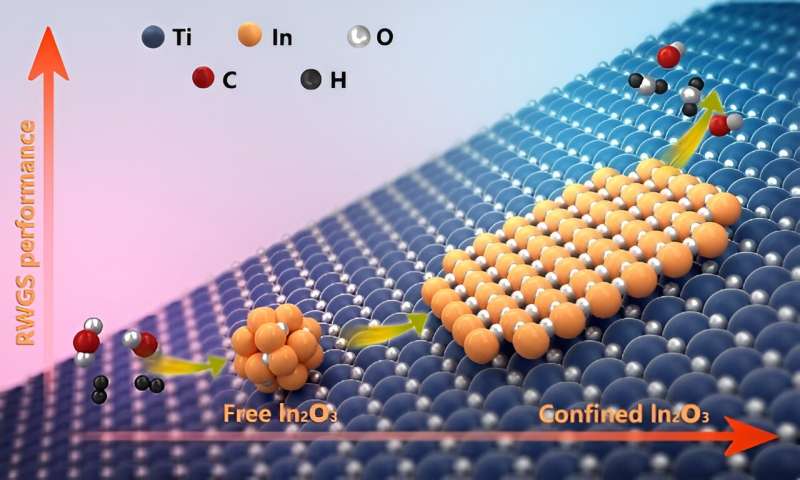
Confined catalysis has been regarded as an important strategy to modulate chemical reactions and enhance catalytic performance. Previous studies have demonstrated that the applications of the confinement effect in catalysis are in enclosed nanospace. However, whether an open space also has this effect is still unclear.
Recently, a research group led by Prof. Bao Xinhe and Prof. Fu Qiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed the interface confinement effect on open space in an In2O3-TiO2 catalyst during a reverse water gas shift (RWGS) reaction. The study is published in Journal of the American Chemical Society.
The researchers physically mixed In2O3 and TiO2 to obtain an In2O3-TiO2 catalyst for the RWGS reaction. They verified that the open surface of TiO2 could create a confined environment for In2O3, which drove the spontaneous transformation of free In2O3 nanoparticles into In oxide nanolayers (InOx) covering onto the TiO2 surface during RWGS.
Additionally, the researchers found that the formed InOx nanolayers were easy to create in surface oxygen vacancies but were against over-reduction to metallic In in the H2-rich atmospheres, resulting in enhanced activity and stability compared with the pure In2O3 catalyst. They identified that the formation of interfacial In–O–Ti bonding drove the In2O3 dispersion and stabilized the metastable InOx layer.
Therefore, the researchers demonstrated that the InOx overlayers with distinct chemistry from their free counterparts could be confined on various oxide surfaces, demonstrating the important confinement effect at oxide–oxide interfaces.
"The interface confinement effect plays an important role in many oxide–oxide catalysts, which can be used to enhance the catalytic performance," said Prof. Fu.
More information: Jianyang Wang et al, Confinement-Induced Indium Oxide Nanolayers Formed on Oxide Support for Enhanced CO2 Hydrogenation Reaction, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c13355
Journal information: Journal of the American Chemical Society
Provided by Chinese Academy of Sciences