This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers report a novel role for S100A11 protein in focal adhesion regulation
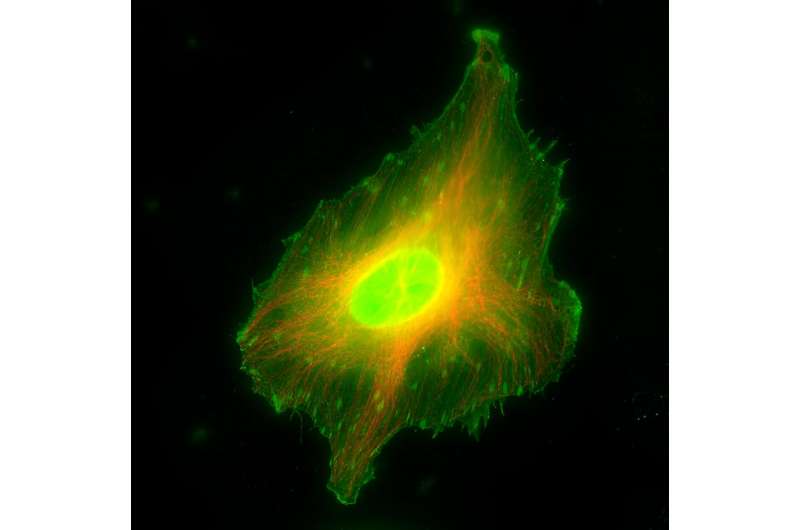
S100A11 is a small Ca2+-activatable protein with an established role in different cellular processes involving actin cytoskeleton remodeling, such as cell migration, membrane protrusion formation, and plasma membrane repair. It also displays F-actin binding activity and localizes to actin stress fibers, but its precise role in regulating these structures has remained unclear.
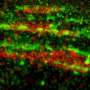
In their study published in the Journal of Cell Science, Tareg Omer Mohammed, You-Rong Lin, and Clemens M. Franz together with colleagues from the Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, Japan, and Karlsruhe Institute of Technology, Germany, report a novel localization of S100A11 to disassembling focal adhesions at the end of contractile stress fibers in HeLa and U2OS cells.
Specifically, S100A11 transiently appears at the onset of focal adhesion disassembly, reliably marking the targeted adhesion sites for subsequent disassembly. Interestingly, S100A11 leaves focal adhesion sites before the completion of disassembly, indicating that S100A11 plays a specific role in the initiation of adhesion site disassembly, rather than the disassembly process itself.
Focal adhesions are integrin-containing cell/matrix adhesion sites enabling cells to adhere to the cellular environment and to apply cellular contraction forces during extracellular matrix remodeling. Directed cell migration requires the coordinated assembly of new adhesion sites at the front, and disassembly at the rear of the cell, and better understanding mechanisms regulating focal adhesion turnover is, therefore, an important goal in cell migration and invasion research. The newly discovered role of S100A11 in focal adhesion disassembly extends the insight into the molecular mechanisms underlying focal adhesion site disassembly.
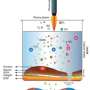
The authors also delineate a force-dependent recruitment mechanism S100A11 to adhesion sites involving non-muscle myosin II-driven stress fiber contraction, activation of mechanosensitive, Ca2+-permeable Piezo1 channels, and intracellular Ca2+ influx at mechanically stressed focal adhesions. In turn, locally elevated Ca2+ levels activate and recruit S100A11 to the adhesion sites targeted for disassembly.
The force-dependent recruitment of S100A11 to stressed focal adhesions was confirmed using a micropipette pulling assay able to apply pulling forces onto individual focal adhesion sites. Even when myosin II-dependent intracellular contractility was inhibited, external pulling forces still recruited S100A11 to stretched focal adhesion sites, corroborating the mechanosensitive recruitment mechanism of S100A11.
However, extracellular Ca2+ and Piezo1 function was still indispensable, indicating that myosin II-dependent contraction forces act upstream of Piezo1-mediated Ca2+ influx, in turn leading to S100A11 activation and FA recruitment.
Lastly, the authors show impaired focal adhesion translocation and disassembly rates in S100A11 knockout cells, confirming the important role of S100A11 in focal adhesion turnover. Together, these results reveal an unsuspected mechanosensitive role of S100A11 during actomyosin contractility-mediated focal adhesion disassembly. The novel identification of S100A11 as a mechano-responsive protein thus further expands its diverse functional repertoire.
Cancer cell migration and invasion crucially depend on focal adhesion modulation and actin-binding proteins are frequently dysregulated in cancer and contribute to malignancy and unfavorable prognosis. S100A11 has a well-established role in cancer, promoting aggressive traits such as enhanced migration, invasion, and metastasis. In this context, the newly identified role of S100A11 in regulating FA disassembly could contribute to its pro-migratory and -invasive properties.
More information: Tareg Omer Mohammed et al, S100A11 promotes focal adhesion disassembly via myosin II-driven contractility and Piezo1-mediated Ca2+ entry, Journal of Cell Science (2024). DOI: 10.1242/jcs.261492
Provided by Kanazawa University