February 29, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Towards the selective and energy-efficient synthesis of ethylene via carbon dioxide reduction
The synthesis of carbon-based chemicals via the electrochemical reduction of carbon dioxide (CO2) has become the key objective of numerous recent energy research efforts. While these studies have yielded promising results, enabling the production of various widely used chemicals, most proposed approaches exhibit poor energy efficiencies and selectivity.
Proposed methods for the electrochemical reduction of CO2 into the hydrocarbon ethylene, for instance, have so far not achieved desirable energy efficiency and stability. This has prevented their widespread deployment as alternatives for conventional petrochemical approaches to produce ethylene, which have adverse effects on the environment.
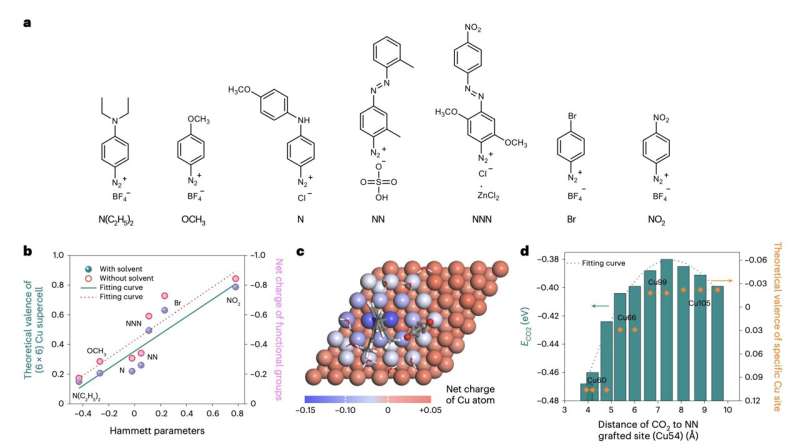
Researchers at Université Montpellier and other institutes recently set out to facilitate the selective and energy-efficient synthesis of ethylene via the reduction of CO2 by functionalizing catalysts that prompt reduction reactions. Their paper, published in Nature Energy, introduces a strategy to functionalize copper (Cu) catalysts for CO2 reduction using aryl diazonium salts, colorless substances currently employed to synthesize various organic compounds.
"Although progress has been made in producing multi-carbon products from the electrochemical reduction of CO2, the modest selectivity for ethylene (C2H4) leads to low energy efficiency and high downstream separation costs," Huali Wu, Lingqi Huang and their colleagues wrote in their paper. "We functionalize Cu catalysts with a variety of substituted aryl diazonium salts to improve selectivity towards multi-carbon products."
In their calculations and experiments, Wu, Huang, and their collaborators found that different aryl diazonium salts could help to tailor the oxidation state of Cu. Using these salts, they were thus able to functionalize catalysts into a membrane electrode assembly (MEA) cell, the primary component of fuel cells that facilitates desired electrochemical reactions, including the reactions underpinning the reduction of CO2.
The researchers tested the performance of this MEA flow cell with tailored Cu sites in a series of experiments. They found that their functionalization strategy enhanced the energy efficiency and stability of CO2 reduction to produce ethylene.
"Using computation and operando spectroscopy, we find that Cu surface oxidation state (δ+ where 0 <� δ <� 1) can be tuned by functionalization and that it influences the selectivity to C2H4," the researchers wrote.
"We report a Faradaic efficiency and a specific current density for C2H4 as large as 83 ± 2% and 212 mA cm−2, respectively, on partially oxidized Cu0.26+. Using a CO gas feed, we demonstrate an energy efficiency of ~40% with a C2H4 Faradaic efficiency of 86 ± 2%, corresponding to a low electrical power consumption of 25.6 kWh Nm−3 for the CO to C2H4 conversion reaction."
The recent study by this team of researchers introduces a new promising strategy to enable the energy-efficient and stable electrosynthesis of ethylene from CO2, leveraging the valence engineering of copper. This strategy could soon be refined and further validated, potentially contributing to the future shift towards more sustainable methods to produce ethylene on a large scale.
More information: Huali Wu et al, Selective and energy-efficient electrosynthesis of ethylene from CO2 by tuning the valence of Cu catalysts through aryl diazonium functionalization, Nature Energy (2024). DOI: 10.1038/s41560-024-01461-6.
Journal information: Nature Energy
© 2024 Science X Network