This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Eco-friendly catalyst and materials research explores pathways to renewable energy
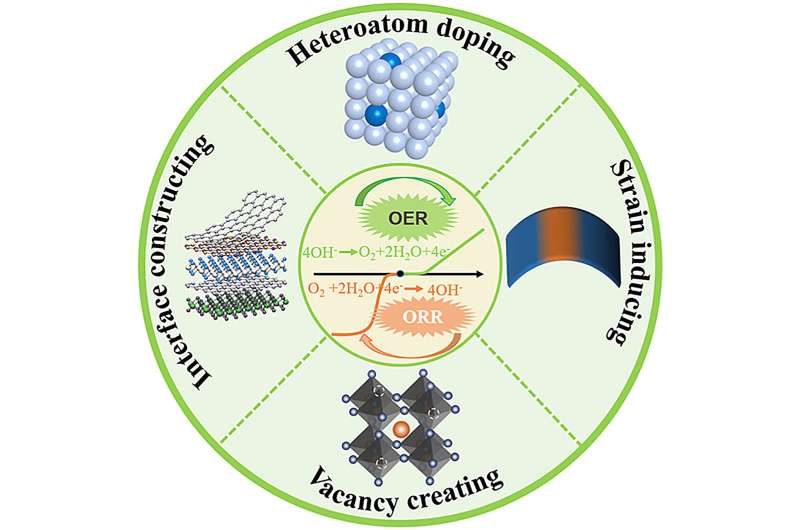
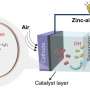
The search for sustainable and affordable energy conversion technologies has highlighted the importance of the oxygen reduction and oxygen evolution reactions (ORR and OER). These processes are crucial for the efficiency of devices such as fuel cells and electrolyzers but have traditionally relied on costly noble metals as catalysts. This dependency poses significant barriers to broader adoption and scalability.
In a review published in the journal eScience, a research team from Technische Universität Dresden have reviewed the major advancement in creating effective and affordable electro-catalysts without the use of noble metals for oxygen electrocatalysis in alkaline electrolytes.
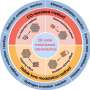
The review provides an in-depth analysis of noble metal-free catalysts, focusing on three primary categories: transition metal compounds, single-atom catalysts, and entirely metal-free options. Researchers utilized several innovative strategies to enhance these catalysts' effectiveness for oxygen electrocatalysis, crucial for energy conversion technologies.
Techniques such as heteroatom doping, which introduces different atoms into the catalyst structure, vacancy creation that alters material properties by removing atoms, and strain induction to modify electronic properties, were key. These approaches aimed to improve the catalysts' physicochemical characteristics, significantly boosting their performance in oxygen reduction and evolution reactions.
This research underscores the potential of noble metal-free materials in replacing costly and scarce noble metals traditionally used in catalysts, highlighting a path towards more affordable and sustainable energy conversion devices.
In addition to exploring improvements in oxygen electrocatalysis via electronic structure regulation of non-noble materials, a recent study published in the same journal by researchers from the University of Technology Sydney and the Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences has made significant advances in materials for energy conversion and storage.
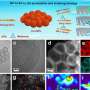
They have introduced a method for synthesizing metal-organic framework (MOF)-derived nanocages, which considerably enhance the efficiency of water oxidation and selective ethylene glycol reformation, marking a notable progress in the field of sustainable energy production.
The studies introduce innovative materials and techniques to improve the efficiency of processes such as water electrolysis for hydrogen production, contributing to the evolution of energy conversion and storage technologies. These efforts align with the broader goal of developing cost-effective, efficient, and environmentally friendly alternatives to conventional energy sources, thereby supporting the global transition towards renewable energy sources.
More information: Xia Wang et al, Electronic structure regulation of noble metal-free materials toward alkaline oxygen electrocatalysis, eScience (2023). DOI: 10.1016/j.esci.2023.100141
Minghong Huang et al, Controlled synthesis of MOF-derived hollow and yolk–shell nanocages for improved water oxidation and selective ethylene glycol reformation, eScience (2023). DOI: 10.1016/j.esci.2023.100118
Provided by TranSpread