A cobalt(II) porphyrin with a tethered imidazole for efficient oxygen reduction and evolution electrocatalysis
Recently, Rui Cao and Weiqiang Zhang of Shaanxi Normal University, Shunichi Fukuzumi and Wonwoo Nam of Ewha Womans University, and others published a letter entitled "A cobalt(II) porphyrin with a tethered imidazole for efficient oxygen reduction and evolution electrocatalysis" in the Journal of Energy Chemistry.
Electrocatalytic oxygen reduction and evolution reactions are employed in various fuel cells, metal–air batteries, and water splitting devices. The large-scale use of noble metals and their complexes in industrial applications is limited by their low earth abundance and high cost. However, several first-row transition metal complexes have recently been identified as active electrocatalysts for the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER). Nevertheless, few of these catalysts are efficient for both the ORR and OER under the same conditions, which is crucial for their use in rechargeable metal–air batteries.
Co porphyrins have also been widely studied as catalysts for O2 reduction. In addition to the ORR, Co porphyrins have been shown to be efficient OER electrocatalysts. Despite these achievements, the development of Co porphyrins with high activity and stability for both the electrocatalytic oxygen reduction and evolution reactions under the same conditions is still needed.
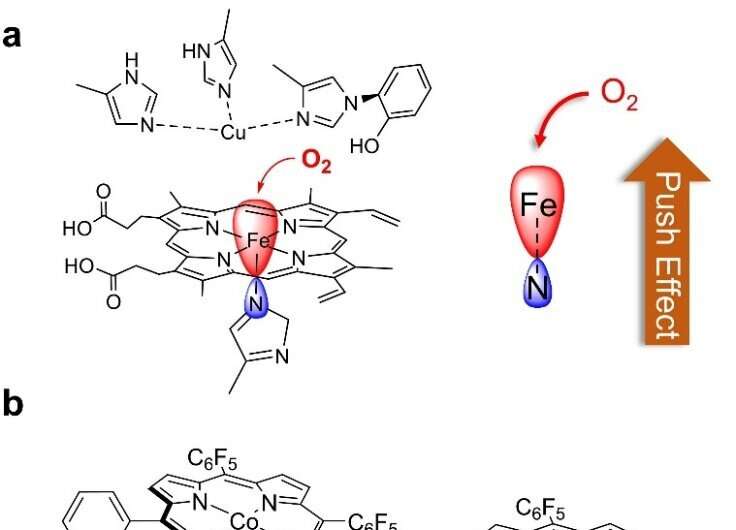
In cytochrome c oxidases (CcOs), Fe porphyrins with an axial histidine imidazole group catalyze the selective reduction of O2 to water. The axial imidazole group increases the electron density of the Fe, thereby enhancing O2 binding and activation at the Fe center through an electronic "push effect" (Fig. 1a).
Inspired by nature, the authors report a Co porphyrin, 1, with an imidazole group appended at the porphyrin backbone for Co axial binding as an active bifunctional electrocatalyst for the oxygen reduction and evolution reactions (Fig.1b). In addition, 1 was employed as the catalyst of the air electrode used to assemble Zn-air batteries.

The resulting Zn-air batteries displayed comparable performance to ones made using commercial Pt/C and Ir/C materials. This work is significant in underlining the important role of axial ligands in enhancing the ORR and OER as well as in showing the potential applications of molecular catalysts in electrocatalysis-based energy conversion and storage technologies.
More information: Xialiang Li et al, A cobalt(II) porphyrin with a tethered imidazole for efficient oxygen reduction and evolution electrocatalysis, Journal of Energy Chemistry (2022). DOI: 10.1016/j.jechem.2022.10.010
Provided by Chinese Academy of Sciences