This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers profile two elusive proteins critical for healthy cell division
The cells in our bodies constantly divide and renew themselves. But if division goes wrong, cancer or other diseases can result. Now, University of Connecticut researchers have profiled two elusive proteins critical for healthy cell division. They report their results in the Dec. 20 issue of Nature.
A dividing cell is like a ballet. Many different dancers move on and off stage performing different roles. If the choreography is off, the ballet dissolves into chaos.
In cell division, enzymes control parts of the precise choreography. UConn School of Medicine researchers were particularly interested in a phosphatase enzyme. This phosphatase has to sit out most of the early action in cell division, but has a critical role to direct later on.
Two proteins block the phosphatase, holding it harmless until its time for action begins. These proteins, called FAM122A and ARPP19, may play critical roles in certain types of cancer and other diseases of cell division. But the two proteins are extremely difficult to study.
"These are two proteins without a form, called intrinsically disordered proteins. They are like dynamic noodles," says Wolfgang Peti, a chemical biologist in the School of Medicine. But even for these proteins, form is critical. When they bind the phosphatase, FAM122A and ARPP19 have postures as precise as a ballerina's arabesque. But until now, nobody had ever caught them in the act.
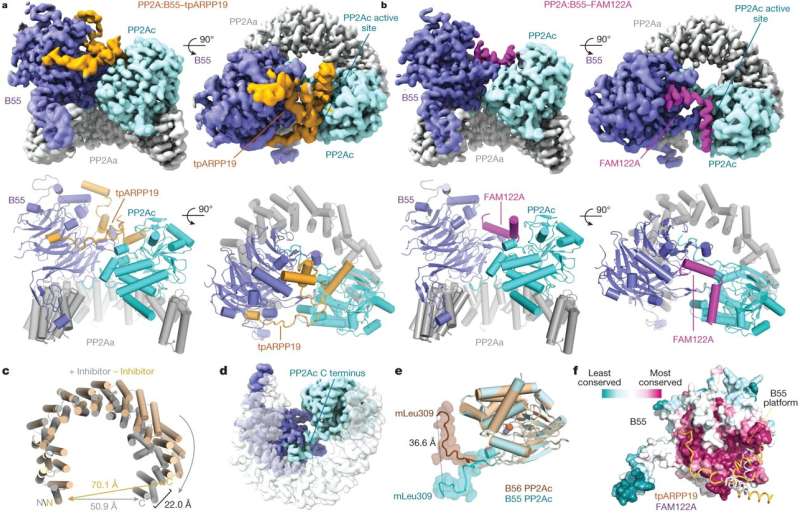
Now, Peti, first author Sathish Padi, and their colleagues can report that they have. The researchers describe the structures of both FAM122A, and ARPP19 when bound to phosphatase PP2A:B55 in their Nature article. Now that they know how the proteins bind to the phosphatase enzyme, they can check mutations observed in cancer cells to see if the mutant protein binds more weakly or strongly, which in turn influences how quickly the cell can divide. Speeding division up might make a cancer grow faster, for example.
Getting the structures of FAM122A and ARPP19 when they were bound to PP2A:B55 was arduous. First the team performed nuclear magnetic resonance (NMR) on the proteins in a liquid solution, to see their general molecular structure—how their atoms are connected to one another. But the proteins in solution are floppy and formless. To understand FAM122A and ARPP19 block the phosphatase activity, the team used cryo-electron microscopy to take pictures of the proteins that were frozen just as they were binding to phosphatase PP2A:B55.
Reconstructing the proteins' shapes was so difficult that it took large amounts of protein, painstakingly purified from hundreds of liters of mammalian cells over five years, and more than 2,000,000 images, to successfully reconstruct the proteins' shapes.
But now that they know those structures, the researchers have a powerful tool.
"Now, we can take mutations from cancer cells, plug them into this protein's structure and boom, immediately know how the protein's function changes," Peti says.
The researchers are currently looking at other phosphatases that are active in the cell growth and division cycle to understand them equally well.
More information: Sathish K. R. Padi et al, Cryo-EM structures of PP2A:B55–FAM122A and PP2A:B55–ARPP19, Nature (2023). DOI: 10.1038/s41586-023-06870-3
Journal information: Nature
Provided by University of Connecticut