This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Pathogen protein modularity enables elaborate mimicry of host phosphatase
Pathogens have developed an extensive array of proteins during the co-evolutionary arms race with their hosts. This is particularly true for Phytophthora, a genus that causes significant damage to agriculture and forestry. One well-known species, Phytophthora infestans, is responsible for the devastating late blight pandemic during the Irish Potato Famine. Each Phytophthora species encodes hundreds of effector proteins that play essential roles in manipulating host cellular processes and promoting disease development.
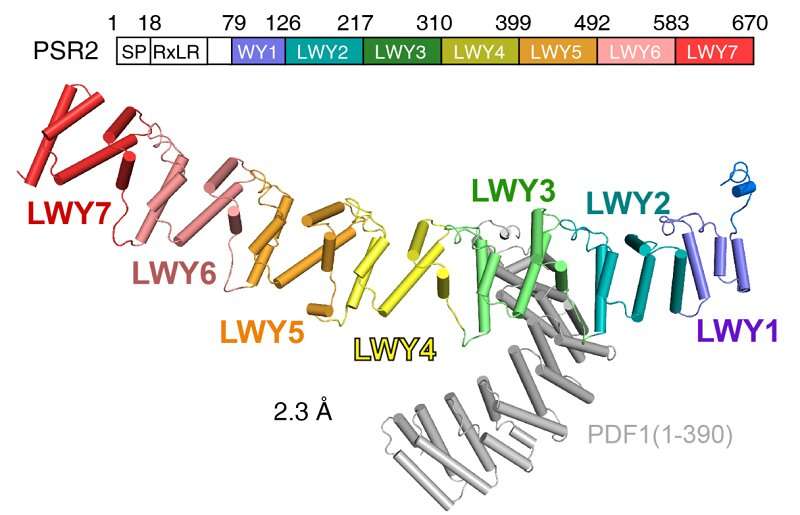
A previous study demonstrated that Phytophthora suppressor of RNA silencing 2 (PSR2) exhibits a conserved modular fold that contributes to its virulence. The (L)WY motifs within PSR2 form distinct structures, which affect the surface residues of effector proteins and likely impact their ability to interact with plant molecules. This raised questions about whether these repeated modules serve specific functions within effector proteins, aiding their evasion of plant immune defense and facilitating their evolution.
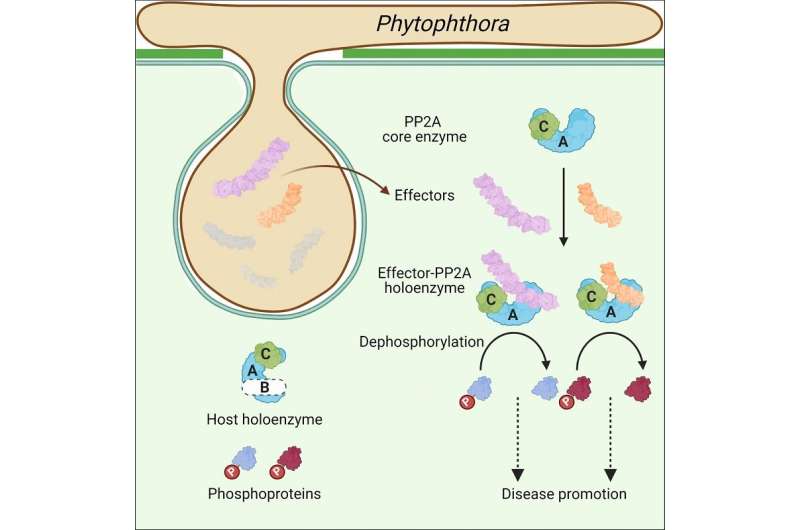
In the new study published in Cell, researchers from the Institute of Biophysics of the Chinese Academy of Sciences and their collaborators found numerous effectors from Phytophthora that possess a conserved PP2A interacting module, mimicking the regulatory subunits of PP2A to hijack the PP2A core enzyme.
To investigate the role of (L)WY units in virulence and effector evolution, the researchers used the PSR2 effector from the soybean pathogen P. sojae as a model. They found that PSR2 interacts with the protein phosphatase 2A (PP2A) enzyme in the host plant, a major phosphatase, and modifies its phosphorylation function to promote disease.
Through crystal structure analysis and biochemical experiments, the researchers revealed a specific LWY2-LWY3 combination within PSR2, referred to as the PP2A interacting module, which competes for the recruitment of the PP2A core enzyme. Through sequence alignment and structural comparison, they observed a high degree of structural and sequence conservation in the amino acid region corresponding to the PP2A interacting module among the identified WY1-(LWY)n effector proteins.
In addition, the researchers selected 15 highly conserved effector proteins for screening, and confirmed that twelve of them also hijack the PP2A core enzyme in host cells.
To validate these findings, the researchers focused on one of the proteins, PITG_15142, and obtained its crystal structure. Structural analysis and in vitro biochemical experiments confirmed the conservation of the PP2A interacting module.
This study shows that (L)WY tandem repeats serve as functional modules in Phytophthora effector, a specific (L)WY-LWY combination enables the hijacking of the host PP2A core enzyme, and multiple effectors adopt this module to hijack the host PP2A core enzyme. It provides the first evidence of how functional diversity and novel virulence activities can arise within a pathogen's effector repertoire, and sheds light on how protein modularity promotes diversity within this repertoire.
More information: Hui Li et al, Pathogen protein modularity enables elaborate mimicry of a host phosphatase, Cell (2023). DOI: 10.1016/j.cell.2023.05.049
Journal information: Cell
Provided by Chinese Academy of Sciences