This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel switch turns genes on/off on cue, a promising step toward safer gene therapy
Just like a doctor adjusts the dose of a medication to the patient's needs, the expression of therapeutic genes, those modified in a person to treat or cure a disease via gene therapy, also needs to be maintained within a therapeutic window. Staying within the therapeutic window is important as too much of the protein could be toxic, and too little could result in a small or no therapeutic effect.
Although the principle of therapeutic window has been known for a long time, there has been no strategy to implement it safely, limiting the potential applications of gene therapy in the clinic.
In their current study published in the journal Nature Biotechnology, researchers at Baylor College of Medicine report on a technology to effectively regulate gene expression, a promising solution to fill this gap in gene therapy clinical applications. A Research Briefing on the breakthrough has been published in the same journal issue.
"Although there are several gene regulation systems used in mammalian cells, none has been approved by the U.S. Food and Drug Administration for clinical applications, mainly because those systems use a regulatory protein that is foreign to the human body, which triggers an immune response against it," said corresponding author Dr. Laising Yen, associate professor of pathology and immunology and of molecular and cellular biology at Baylor.
"This means that the cells that are expressing the therapeutic protein would be attacked, eliminated or neutralized by the patient's immune system, making the therapy ineffective."
For more than a decade, Yen and his colleagues have been working on this technology and now they have found a solution to overcome the main obstacles in its clinical use. "The solution we found does not involve a foreign regulatory protein that will evoke an immune response in patients. Instead, we use small molecules to interact with RNA, which typically do not trigger an immune response," Yen said.
"Other groups also have made attempts to resolve this critical issue, but the drug concentrations they used are beyond what the FDA has approved for patients. We were able to engineer our system in such a way that it works at the FDA-approved dosage."
A switch to turn genes on/off on cue
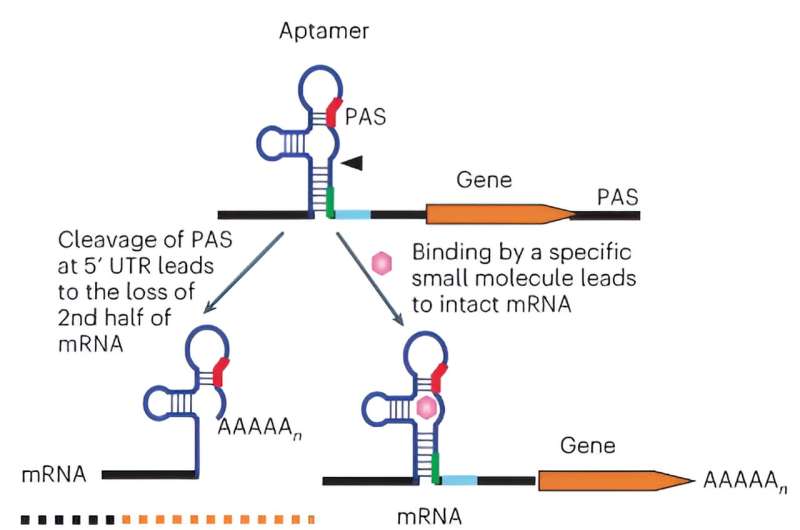
Yen and his colleagues developed a system that turns genes on to different levels on cue using small molecules at FDA-approved doses. The switch is placed in the RNA, the copy of genetic material that is translated into a protein. This approach allows the researchers to control the protein's production a step back by controlling its RNA.
The RNA of interest is first engineered to contain an extra polyA signal, akin to a "stop sign" that genes naturally use to mark the end of a gene. When the machinery of the cell detects a polyA signal in the RNA, it automatically makes a cut and defines the cut point as the end of the RNA. "In our system, we use the added polyA signal, not at the end, but at the beginning of the RNA, so the cut destroys the RNA and therefore the default is no protein production. It is turned off until we turn it on with the small molecule," Yen said.
To turn on the gene at the desired level, the team engineered a switch on the RNA. They modified a section of the RNA near the polyA signal such that it can now bind to a small molecule, FDA-approved tetracycline in this case. "When tetracycline binds to that section that functions as a sensor on the RNA, it masks off the polyA signal, and the RNA will now be translated into protein," Yen said.
Imagine the now possible future situation. A patient has received gene therapy that provides a gene to compensate for a malfunctioning gene that causes a medical condition. The gene the patient received has the switch, which allows the physician to control the production of the therapeutic protein.
If the patient only requires a small amount of the therapeutic protein, then he/she will only take a small dose of tetracycline, which will turn on the therapeutic gene only a little. If the patient needs more therapeutic protein, then he/she would take more tetracycline to boost production. To stop production of the therapeutic protein, the patient stops taking tetracycline. In the absence of tetracycline, the switch will be back to its default off position.
Some diseases may benefit from the presence of constant low levels of therapeutic protein. In that case, the technology has the flexibility to pre-adjust the default level to specified levels of protein expression while retaining the option of dialing up the expression with tetracycline.
"This strategy allows us to be more precise in the control of gene expression of a therapeutic protein. It enables us to adjust its production according to disease's stages or tune to the patients' specific needs, all using the FDA-approved tetracycline dose," Yen said. "Our approach is not disease-specific, it can theoretically be used for regulating the expression of any protein, and potentially has many therapeutic applications.
"In addition, this system is more compact and easier to implement than the existing technologies. Therefore, it also can be very useful in the lab to turn a gene of interest on or off to study its function."
More information: Liming Luo et al, Control of mammalian gene expression by modulation of polyA signal cleavage at 5′ UTR, Nature Biotechnology (2024). DOI: 10.1038/s41587-023-01989-0.
A step closer to safe and efficient mammalian gene regulation, Nature Biotechnology (2024). DOI: 10.1038/s41587-023-02020-2
Journal information: Nature Biotechnology
Provided by Baylor College of Medicine