This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel approaches for correcting gene expression insufficiency
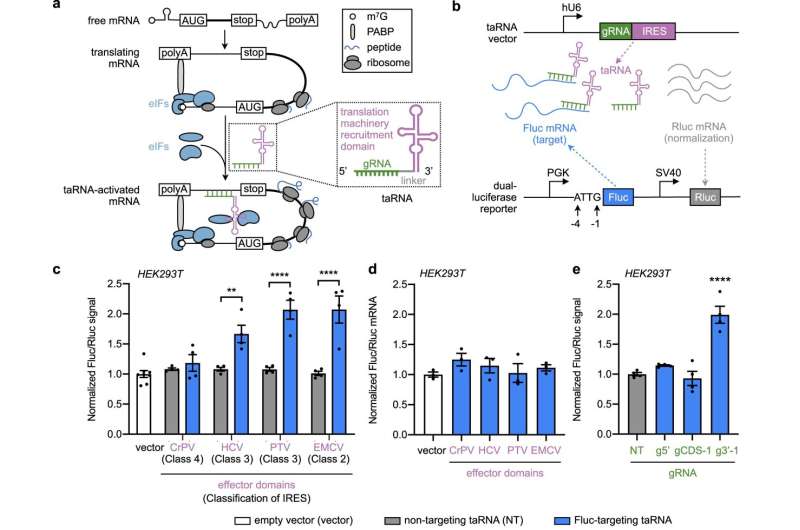
A new molecular technology capable of binding to mRNA and regulating gene expression may offer a new avenue for treating diseases caused by haploinsufficiency, or the absence of one functional gene copy, according to a study published in Nature Communications.
Messenger RNA, or mRNA, contains instructions for DNA to produce proteins. Many diseases, including cancer and many genetic disorders, result from insufficient gene—and therefore protein—expression, but few strategies exist to correct that kind of dysregulation at a molecular level.
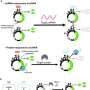
The new technology, dubbed "translation-activating RNAs" (taRNAs), consists of small molecules programmed to attach to specific mRNA molecules to directly control their translation into proteins, said Alfred L. George Jr., MD, the chair and Alfred Newton Richards Professor of Pharmacology and a co-author of the study.
"This technology opens the door for a new way to enhance the expression of a gene at the protein level. There are lots of ways to knock down a gene. There are very few ways to upregulate the gene and produce more protein from the copy of the gene that's not disabled by a mutation," said George, who also directs the Center for Pharmacogenomics.
The study was a collaborative effort between George and Bryan Dickinson, Ph.D., professor of Chemistry at the University of Chicago, who was senior author of the paper.
In the study, investigators combined snippets of sequences within RNA molecules to create taRNAs that could target PTEN, an mRNA known to act as a potent tumor-suppressor, and ABCA7, an mRNA often found to be deficient in patients with Alzheimer's disease. After administering the taRNAs to cultured human cells, investigators found that the levels of both mRNAs had increased.
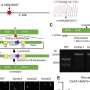
Investigators then programmed mRNAs to bind to PTEN and CDKN1A, another gene linked to tumor suppression, in a human sample of aggressive triple-negative breast cancer. Following an injection of taRNAs, tumor growth slowed by half, according to the study.
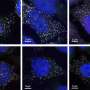
George and his collaborators then utilized the taRNAs to stimulate the production of SYNGAP1 in human neurons. SYNGAP1 is a major regulator of synaptic activity, and children born with only one functional copy of this gene suffer from epilepsy and severely impaired neurodevelopment.
Human neurons generated from patient-derived induced pluripotent stem cells in the George laboratory that received the taRNAs showed restoration of the SYNGAP1 protein to normal levels, according to the study.
The findings of the study offer a promising new strategy for treating a variety of diseases stemming from gene and protein expression insufficiency, George said.
"The fact that this approach worked in human neurons is very exciting," George said. "There are many technologies that work in the laboratory in artificial systems, but don't work as well in human cells. Human neurons are particularly challenging."
The study is a great example of institutional collaboration powering new discoveries, George said.
"Faculty at Northwestern have invested a lot of effort to develop cell lines from children with rare neurogenetic and neurodevelopmental disorders, like SYNGAP1-related intellectual disability. This cell resource is a goldmine for testing novel therapeutic strategies, like the one featured in this paper," George said.
"This study is good example of collaboration between Chicagoland institutions as well as between a chemical biologist and my laboratory, which investigates human disorders with stem cell models of the disease."
More information: Yang Cao et al, RNA-based translation activators for targeted gene upregulation, Nature Communications (2023). DOI: 10.1038/s41467-023-42252-z
Journal information: Nature Communications
Provided by Northwestern University