This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Zooplankton in ocean and freshwater are rapidly escalating the global environmental threat of plastics, finds study
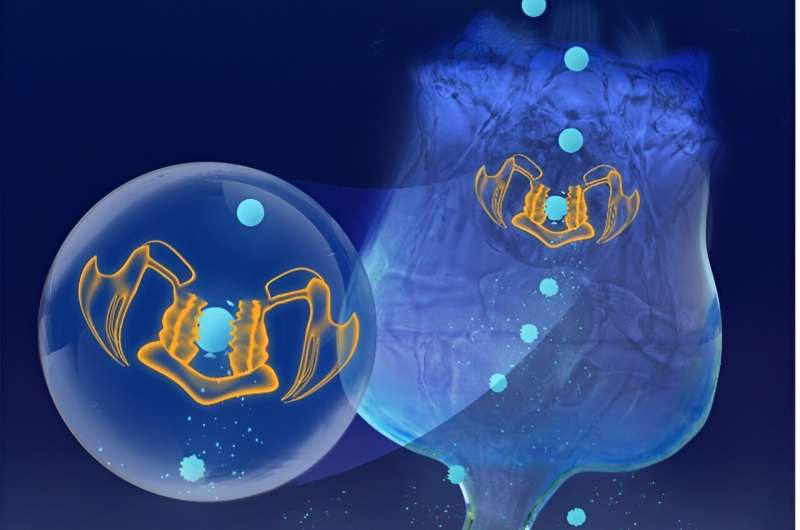
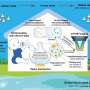
A collaborative research team lead by the University of Massachusetts Amherst has recently revealed that rotifers, a kind of microscopic zooplankton common in both fresh and ocean water around the world, are able to chew apart microplastics, breaking them down into even smaller, and potentially more dangerous, nanoplastics—or particles smaller than one micron. Each rotifer can create between 348,000–366,000 per day, leading to uncountable swarms of nanoparticles in our environment.
In China's Poyang Lake, the country's largest freshwater lake, the researchers calculated that rotifers were creating 13.3 quadrillion particles every single day. The research was reported in Nature Nanotechnology.
It is well known that plastic is an incredibly durable material, which can take up to 500 years to decompose. As plastic bottles, packaging and parts get older, teeny pieces of them break off. These microplastics have been found in every corner of the globe, from the top of Mount Everest to the depths of the Mariana Trench, and, according to recent reports, they are in many humans' blood and heart tissue.
The problem is that microplastics pose an as-of-yet unknown risk to the environment and to human health, and they're altering ecosystems throughout the world.
The smaller the plastic particle, the more easily they spread and the more of them they are. Each individual microplastic could theoretically be broken down into 1,000,000,000,000,000 nanoplastic particles.
Smaller size also means more surface area, which means they are more reactive and potentially even more harmful to the health of humans and other living beings than microplastics. While there has been much attention given to microplastics, there has been far little interest in studying nanoplastics, particularly in how they're generated, which means we don't really know how many nanoplastics might be out there.
"Humans produce enormous amounts of plastics, and yet we don't have an effective way of recycling them," says Baoshan Xing, University Distinguished Professor of Environmental & Soil Chemistry at UMass Amherst's Stockbridge School of Agriculture and the paper's senior author. "We began to wonder about nanoplastics and especially how they're produced."
In part, they're produced through physical and chemical processes: sunlight breaks plastics down, and waves grind bits of plastic against rocks, beaches and other trash floating in the ocean. But Xing and his colleagues wondered what role living creatures might play in the creation of microplastics, especially after learning that Antarctic krill seem to be able to break microplastics down into smaller particles.
Xing and his colleagues were particularly curious about rotifers, of which there are around 2,000 different species worldwide. "Whereas Antarctic krill live in a place that is essentially unpopulated, we chose rotifers in part because they occur throughout the world's temperate and tropical zones, where people live," says Xing.
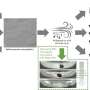
They are abundant—one of the lakes other researchers reported had approximately 23,000 individual rotifers in every liter of water. And rotifers also have a specialized masticatory apparatus—"teeth"—that the team hypothesized could grind microplastics into smaller particles.
After exposing both marine and freshwater species of rotifers to a variety of different plastics of different sizes, they found that all rotifers could ingest microplastics of up to 10 micrometers in size, break them down and then excrete thousands of nanoplastics back into the environment. They estimated that rotifers could produce 13.3 quadrillion nanoparticles every day in Poyang Lake. Scale this up to all of the ocean and fresh bodies of water where both microplastics and rotifers are present, and the number of nanoplastic particles created every day is mind boggling.
"We show for the first time the ubiquitous fragmentation of microplastics by rotifers," says Jian Zhao, professor of environmental science and engineering at the Ocean University of China and the paper's lead author.
"This is a newly discovered route to produce and generate nanoplastics in both freshwater and seawater system worldwide, in addition to well-known physical and photochemical fragmentations. This finding is helpful for accurately evaluating the global flux of nanoplastics. In addition, it is known that nanoplastics can not only be potentially toxic to various organisms, they can also serve as carriers for other contaminants in the environment. Furthermore, the release of chemical additives in the plastic can be enhanced during and after the fragmentation."
"Our work is just the first step," adds Xing. "We need to look at other organisms on the land and in water for biological fragmentation of microplastics and collaborate with toxicologists and public health researchers to determine what this plague of nanoplastics is doing to us."
More information: Microplastic fragmentation by rotifers in aquatic ecosystems contributes to global nanoplastic pollution, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01534-9. www.nature.com/articles/s41565-023-01534-9
Journal information: Nature Nanotechnology
Provided by University of Massachusetts Amherst