This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research dives into the social network of proteins
Stanley Milgram's groundbreaking "Six Degrees of Separation" experiment demonstrated the surprisingly close connections among humans back in the 1960s. Now the research team led by Professor Matthias Mann at the Max Planck Institute (MPI) of Biochemistry has shown that the proteins in our cells are equally well connected.
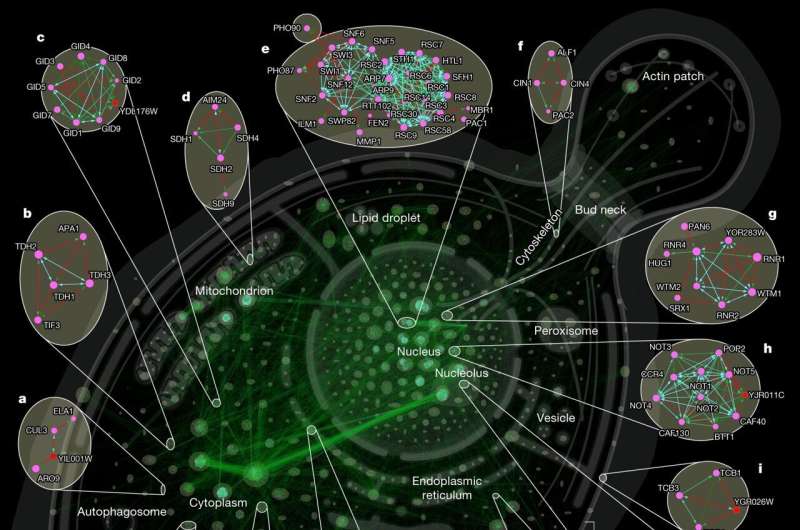
The results of the current study, which are published in the journal Nature, are a decisive step forward in protein research and represent the first comprehensive view of the protein–protein interaction network of an organism.
Milgram's famous experiment from the 1960s showed that, on average, people know each other through only six personal connections. More recent analyses of social networks such as Facebook have shown that we are even more closely connected than originally assumed, with an average of only four and a half "handshakes" between us.
Imagine each protein in our cells as a kind of node in a huge social network, like individuals in a society. Just like between people in a society, the connections and exchanges that proteins make are crucial. What's more, these interactions determine, for example, whether our proteins can perform their functions and tasks properly and therefore whether we stay healthy or not.
In order to study these interactions and the resulting network in our body, scientists have so far only had methods at their disposal that were very incomplete. This made it almost impossible to obtain in-depth analyses and meaningful results about the social network of proteins.
The fisherman's method
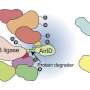
To dive into this social network of proteins, the team at the MPI of Biochemistry used an innovative approach that makes it possible to filter out individual nodes from the network and investigate which other nodes they interact with. The method is acronymized as AP-MS and combines mass spectrometry with affinity purification, a method in which the target protein whose relationships are to be investigated is fished out of the sea of all proteins, so to speak.
The affinity to the proteins with which the target protein interacts directly causes them to be pulled out as well. The mass spectrometer can then measure exactly which proteins are involved. In this way, these proteins can be identified as direct interaction partners of the target protein.
Similar to a fisherman who selects the bait according to his preferred prey, the researchers mark the target protein (bait) with a small, fluorescent protein (fishing rod), which in turn is pulled out of the proteome (sea) by the antibody acting as an angler, dragging the other proteins (fish) with it.
Minimum input, maximum output
In the current study, the researchers succeeded in reducing the previously large amount of 4 liters of cell material to just 1.5 ml. Since it takes a certain amount of time for living cells to grow in a cell culture, the new approach of this study enables a much faster and more precise investigation of the protein network in cells. The new workflow thus offers more in-depth results and a mapped representation of the protein architecture with less cell input and thus less time required.
The study underscores the crucial role of yeast as a model organism due to its comparability to humans. Although yeasts are among the simplest forms of life in nature, they share important cellular functions with us. The molecular interactions of yeasts show that the vast majority of proteins are closely linked, with each protein forming an average of 16 relationships, leaving them only about four "handshakes" apart. Proteins therefore appear to act as socially as we humans do in our social network.
In health and disease
"In order to understand where exactly things go wrong in diseased cells, it is essential to understand how proteins interact with each other in healthy cells. In our study, we were able to discover many new connections between proteins that also occur in human cells and are associated with diseases such as cancer or Huntington's disease," explains André Michaelis, first author of the study.
These investigations into the interaction partners of proteins that are crucial for our health could also be used to draw conclusions about possible new therapeutic approaches in the future. This would make it possible to take a much more individualized approach to the origin of diseases in each person.
Matthias Mann concludes, "The findings of our study have given us an unprecedented insight into the protein network of our cells. I am convinced that the connections and interactions we have discovered will be of crucial importance in the future for the treatment of diseases caused by faulty protein interactions. An exciting project with incredibly detailed results."
More information: André C. Michaelis et al, The social and structural architecture of the yeast protein interactome, Nature (2023). DOI: 10.1038/s41586-023-06739-5
Journal information: Nature
Provided by Max Planck Society