Researchers directly measure electrostatic interactions in protein structures in cells
Electrostatic interaction plays a vital role in many important biological reactions, such as enzyme catalysis, protein-protein interaction, protein-DNA/RNA interaction, and H+ transfer.
Most proteins perform their functions in cells. The complex cellular environment can perturb the electrostatic interactions of proteins. But how the cellular environment modulates protein electrostatic interactions is still unclear.
Recently, a research team from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) has investigated the protein electrostatic interactions in E. coli cells by Nuclear Magnetic Resonance Spectroscopy.
The study was published in Journal of the American Chemical Society on Nov. 12.
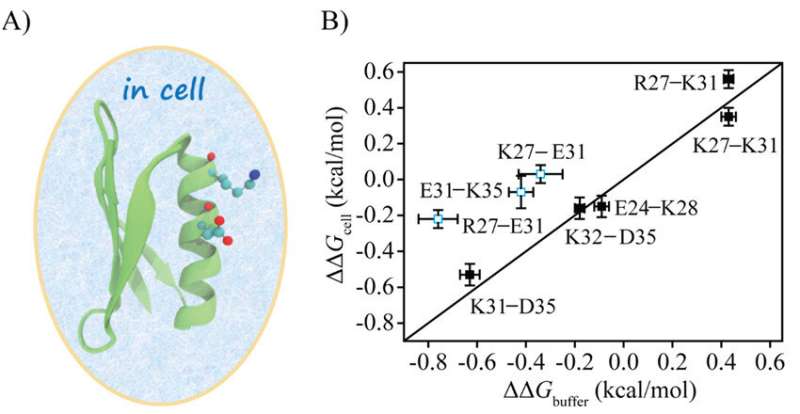
The researchers directly measured the electrostatic interactions between protein charges in cells using the double mutational cycle (DMC) method and compared the results with those measured in a buffer to understand the effect of cellular environment on protein charge interactions.
They introduced eight charge pairs in protein GB3. Compared to the charge pair electrostatic interactions in buffer, five charge pairs in cells displayed no apparent changes whereas three pairs had the interactions weakened by more than 70%.
For the E31-K35 charge pair, the main perturbation was from the folded state, whereas for R27-E31, the main perturbation was from the unfolded state. E. coli cell lysate could capture the weakening of electrostatic interaction of E31-K35 but not that of R27-E31, indicating that lysate can mimic a certain type of interactions in cells.
Further investigation suggested that the transfer free energy was responsible for the electrostatic interaction modulation. Both the transfer free energy of the folded state and that of the unfolded state could contribute to the cellular environmental effect on protein electrostatics although the latter was generally larger (more negative) than the former. That is to say, the cellular environment prefers to interact with the unfolded state more than the folded state. These quinary interactions can weaken electrostatic interactions between certain protein charge pairs.
More information: Xiangfei Song et al, Quantifying Protein Electrostatic Interactions in Cells by Nuclear Magnetic Resonance Spectroscopy, Journal of the American Chemical Society (2021). DOI: 10.1021/jacs.1c10154
Journal information: Journal of the American Chemical Society
Provided by Chinese Academy of Sciences