This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The Goldilocks effect: Researchers establish framework for protein regulation
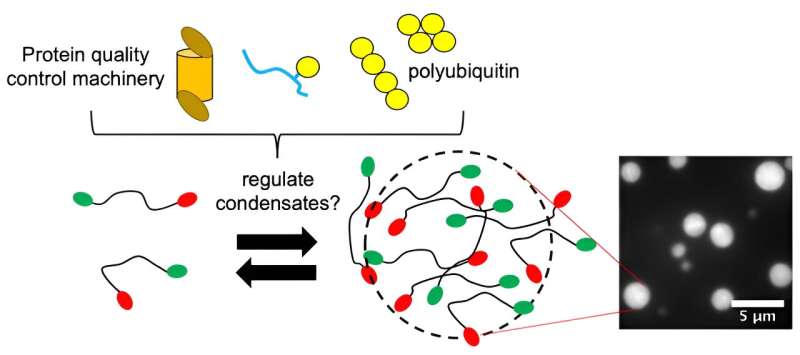
From plants to animals, all living things depend on proteins to help their cells function properly. In certain cases, like when under stress in response to heat or toxins, some proteins within the cell condense into liquid-like droplets called condensates.
This process is hypothesized to occur via phase separation and provides a quick way for the cell to assemble certain components. Research in Syracuse University Professor Carlos Castañeda's lab has recently shown that protein quality control (PQC) components are important for many of these condensates.
Castañeda, associate professor of biology and chemistry in the College of Arts and Sciences (A&S), is among a team of researchers working to understand how protein quality control works in cells. Similar to the way computers use coding as a set of instructions, the PQC receives its instructions from polyubiquitin chains. Ubiquitin (Ub) is a regulatory protein found in all eukaryotic cells (cells containing a defined nucleus) and polyubiquitin is an assembly containing at least a few ubiquitin molecules.
Under normal operating conditions, condensates form and react to a stressor, and then dissolve after the stress is relieved. But when this system becomes disrupted, it can result in protein clumping or aggregation, which can cause cells, especially those in the nervous system, to die.
These abnormal protein aggregates are markers for neurological diseases like amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease. By understanding the circumstances that lead to dysregulation of the PQC, scientists hope this research will one day lead to a cure for such neurodegenerative diseases.
In a paper published last year in EMBO Reports, Castañeda and his collaborators established a framework for how polyubiquitin chains regulate the formation and disassembly of a condensate made of Ubiquilin-2 (UBQLN2) proteins, whose dysregulation is implicated in ALS.
Polyubiquitin is important for UBQLN2 function and the two bind noncovalently, meaning that the interaction between them is weaker than a covalent chemical bond. Following up on that work, the researchers wanted to further explore the specific conditions that affect assembly of these condensates.
"While working on the EMBO Reports project, we started to see that more extended polyubiquitin chains favored phase separation (condensate formation) with UBQLN2," says Castañeda. "So, we wondered if this is always true. We decided to make even more extended chains."
Through the Research Experience for Undergraduates (REU) program, Castañeda welcomed students Suzanne Enos and Antara Chaudhuri into his lab in 2021. The NSF-sponsored REU program brings undergraduate students from across the country to Syracuse's campus to participate in research over the summer.
In Castañeda's lab, the team engineered and designed different types of polyubiquitin chains with variable lengths and topologies. Enos and Chaudhuri then helped test how well these chains phase separated with UBQLN2.
After further design-work by Sarasi Galagedera, a former postdoctoral researcher in Castañeda's lab; Thuy Dao, a lab manager in the Department of Chemistry; and Jeremy Schmit, a professor of physics at Kansas State University, the team found there was a "sweet spot"—or specific spacing between Ub units—where condensate formation was optimized. Fittingly coined as "Goldilocks," the group's findings were recently published in Proceedings of the National Academy of Sciences.
"We found that there is an arrangement of ubiquitin units in polyubiquitin that is 'just right' for condensates to form," says Castañeda. "Ubiquitin units that are too far apart or too close together don't favor condensate formation as much. Jeremy Schmit used theoretical modeling and polyphasic linkage concepts to generalize these experimental observations."
Furthermore, they uncovered that polyubiquitin in excess causes the condensates to disassemble. "In a cell, you can imagine that concentrations of polyubiquitin, as well as the spacing between ubiquitin units within different types of polyubiquitin, can up- or down-regulate condensate formation. You essentially have multiple ways to tune condensate formation with just adding this one polyubiquitin molecule," notes Castañeda.
While their research merely scratches the surface of how polyubiquitin chains can regulate phase separation of condensates, Castañeda says it offers proof that these chains will be a main regulator of droplets. Future studies will involve adapting their rules to an in vitro system that models PQC to prove and test their theories in living cells.
"This work provides a principle that can be applied to understanding how biomolecular condensates are generally controlled and will have large implications for anyone studying the regulation of their favorite biomolecular condensate," says Castañeda.
More information: Sarasi K. K. Galagedera et al, Polyubiquitin ligand-induced phase transitions are optimized by spacing between ubiquitin units, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2306638120
Journal information: Proceedings of the National Academy of Sciences , EMBO Reports
Provided by Syracuse University