Phase separation found in immune response within cells
Protein complexes that play a critical role in launching an immune response assemble in droplets that form within the liquid environment in cells much like oil droplets in water, UT Southwestern scientists report in a new study. The findings, published in Molecular Cell, could lead to new interventions to regulate immunity in individuals with overactive or underactive immune responses.
"These droplets basically function as microreactors that concentrate proteins and their substrates within. It's like forming compartments without needing membranes to surround them," said study leader Zhijian "James" Chen, Ph.D., Professor of Molecular Biology and Director of the Center for Inflammation Research at UTSW, a Howard Hughes Medical Institute Investigator, and winner of the 2019 Breakthrough Prize in Life Sciences.
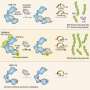
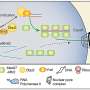
More than two decades ago, the Chen lab discovered that a protein called ubiquitin assembles into chains inside cells when the cells are exposed to inflammatory molecules, such as interleukin-1β (IL-1β) or tumor necrosis factor α (TNFα). Dr. Chen and his colleagues showed that the chains are key for promoting an immune response and can activate a group of proteins known as the IκB complex (IKK), which includes a component known as NEMO. This complex in turn triggers a protein called NF-κB to move to the nucleus and turn on hundreds of immune-related genes. But how the polyubiquitin chains, NEMO, and IKK come together has been unclear.
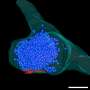
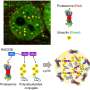
To answer this question, Dr. Chen's team mixed ubiquitin and NEMO in test tubes with a protein called TRAF6, which promotes ubiquitin to assemble into chains. They saw that NEMO and the polyubiquitin chains assembled into liquid droplets that stayed separate from the liquid medium in the test tubes. Experiments in human cells showed that NEMO and the polyubiquitin chains displayed the same "phase separation" behavior after the cells were exposed to IL-1β or TNFα. When IKK entered these droplets, it became activated and triggered NF-κB to move to the nucleus. The longer the polyubiquitin chains, the larger the droplets they formed with NEMO and the stronger the immune response they triggered, Dr. Chen explained.
The team further studied this process using NEMO that was altered by mutations associated with a rare disease known as NEMO deficiency syndrome, which severely blunts immune response to bacterial infections. NEMO that carried these mutations could not effectively condense into droplets with polyubiquitin chains, preventing the cascade of events that triggers an immune response.
Dr. Chen noted that better understanding of this liquid phase separation phenomenon could eventually lead to treatments for NEMO deficiency syndrome and interventions to counteract overactive or underactive immunity, the root cause of autoimmune disorders and increased susceptibility to infection, respectively.
More information: Mingjian Du et al, Liquid phase separation of NEMO induced by polyubiquitin chains activates NF-κB, Molecular Cell (2022). DOI: 10.1016/j.molcel.2022.03.037
Journal information: Molecular Cell
Provided by UT Southwestern Medical Center